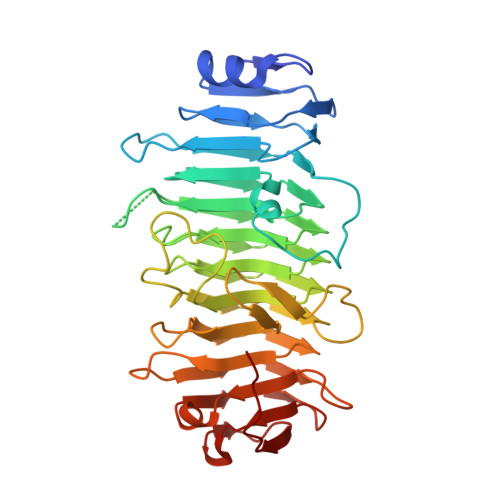
Structure of a Reptilian Adenovirus Reveals a Phage Tailspike Fold Stabilizing a Vertebrate Virus Capsid.
Menendez-Conejero, R., Nguyen, T.H., Singh, A.K., Condezo, G.N., Marschang, R.E., van Raaij, M.J., San Martin, C.(2017) Structure 25: 1562-1573
- PubMed: 28943338
- DOI: https://doi.org/10.1016/j.str.2017.08.007
- Primary Citation of Related Structures:
5G5N, 5G5O - PubMed Abstract:
Although non-human adenoviruses (AdVs) might offer solutions to problems posed by human AdVs as therapeutic vectors, little is known about their basic biology. In particular, there are no structural studies on the complete virion of any AdV with a non-mammalian host. We combine mass spectrometry, cryo-electron microscopy, and protein crystallography to characterize the composition and structure of a snake AdV (SnAdV-1, Atadenovirus genus). SnAdV-1 particles contain the genus-specific proteins LH3, p32k, and LH2, a previously unrecognized structural component. Remarkably, the cementing protein LH3 has a trimeric β helix fold typical of bacteriophage host attachment proteins. The organization of minor coat proteins differs from that in human AdVs, correlating with higher thermostability in SnAdV-1. These findings add a new piece to the intriguing puzzle of virus evolution, hint at the use of cell entry pathways different from those in human AdVs, and will help development of new, thermostable SnAdV-1-based vectors.
Organizational Affiliation:
Departamento de Estructura de Macromoléculas, Centro Nacional de Biotecnología (CNB-CSIC), Darwin 3, 28049 Madrid, Spain.