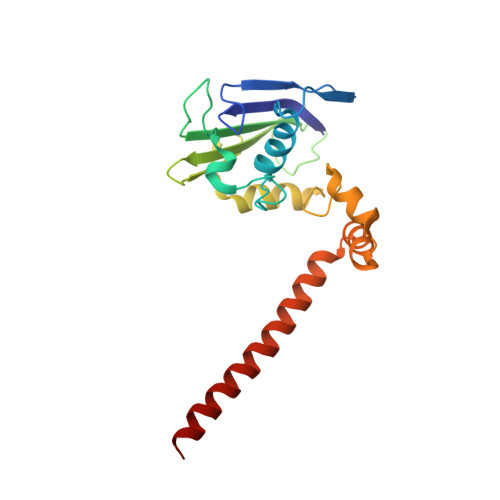
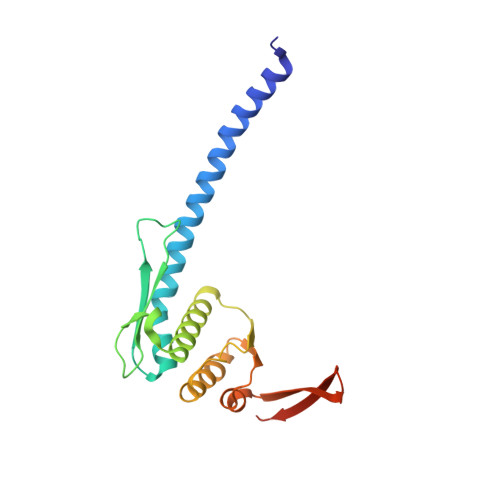
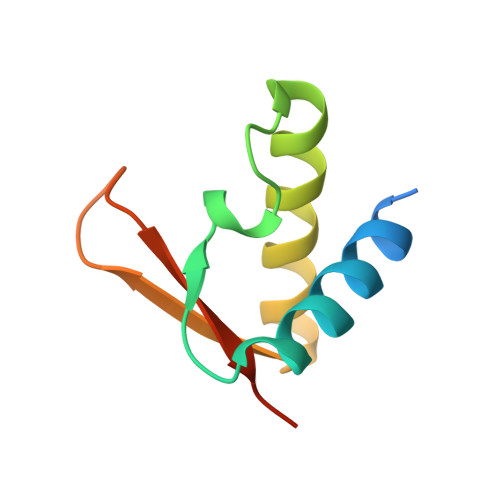
Overall Shapes of the SMC-ScpAB Complex Are Determined by Balance between Constraint and Relaxation of Its Structural Parts
Kamada, K., Su'etsugu, M., Takada, H., Miyata, M., Hirano, T.(2017) Structure 25: 603-616.e4
- PubMed: 28286005
- DOI: https://doi.org/10.1016/j.str.2017.02.008
- Primary Citation of Related Structures:
5H66, 5H67, 5H68, 5H69 - PubMed Abstract:
The SMC-ScpAB complex plays a crucial role in chromosome organization and segregation in many bacteria. It is composed of a V-shaped SMC dimer and an ScpAB subcomplex that bridges the two Structural Maintenance of Chromosomes (SMC) head domains. Despite its functional significance, the mechanistic details of SMC-ScpAB remain obscure. Here we provide evidence that ATP-dependent head-head engagement induces a lever movement of the SMC neck region, which might help to separate juxtaposed coiled-coil arms. Binding of the ScpA N-terminal domain (NTD) to the SMC neck region is negatively regulated by the ScpB C-terminal domain. Mutations in the ScpA NTD compromise this regulation and profoundly affect the overall shape of the complex. The SMC hinge domain is structurally relaxed when free from coiled-coil juxtaposition. Taken together, we propose that the structural parts of SMC-ScpAB are subjected to the balance between constraint and relaxation, cooperating to modulate dynamic conformational changes of the whole complex.
Organizational Affiliation:
Chromosome Dynamics Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan. Electronic address: kamadak@riken.jp.