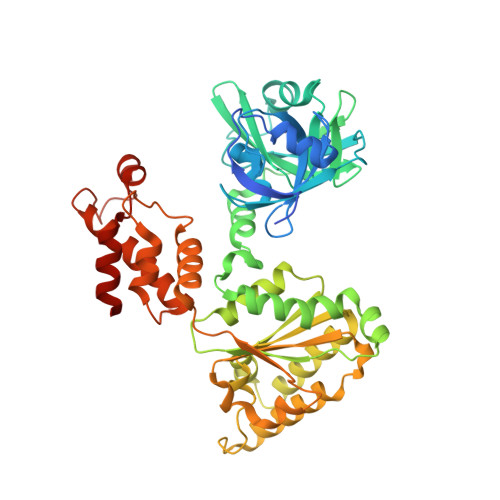
Structural basis for nucleotide-modulated p97 association with the ER membrane.
Tang, W.K., Zhang, T., Ye, Y., Xia, D.(2017) Cell Discov 3: 17045-17045
- PubMed: 29238611
- DOI: https://doi.org/10.1038/celldisc.2017.45
- Primary Citation of Related Structures:
5KIU, 5KIW, 5KIY - PubMed Abstract:
Association of the cytosolic AAA (ATPases associated with various cellular activities) protein p97 to membranes is essential for various cellular processes including endoplasmic reticulum (ER)-associated degradation. The p97 consists of two ATPase domains and an N domain that interacts with numerous cofactors. The N domain of p97 is known to undergo a large nucleotide-dependent conformation switch, but its physiological relevance is unclear. Here we show p97 is recruited to canine ER membranes predominantly by interacting with VCP-interacting membrane protein (VIMP), an ER-resident protein. We found that the recruitment is modulated through a nucleotide-dependent conformation switch of the N domain in wild-type p97, but this modulation is absent in pathogenic mutants. We demonstrate the molecular mechanism of the modulation by a series of structures of p97, VIMP and their complexes and suggest a physiological role of the nucleotide-dependent N domain conformation switch. The lack of modulation in pathogenic mutants is caused by changes in interactions between the N and D1 domain, as demonstrated by multiple intermediate positions adopted by N domains of mutant p97. Our findings suggest the nucleotide-modulated membrane association may also have a role in other p97-dependent processes.
- Laboratory of Cell Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Organizational Affiliation: