Pharmaceutical Characterization of Tropomyosin Receptor Kinase B-Agonistic Antibodies on Human Induced Pluripotent Stem (hiPS) Cell-Derived Neurons.
Traub, S., Stahl, H., Rosenbrock, H., Simon, E., Florin, L., Hospach, L., Horer, S., Heilker, R.(2017) J Pharmacol Exp Ther 361: 355-365
- PubMed: 28351853
- DOI: https://doi.org/10.1124/jpet.117.240184
- Primary Citation of Related Structures:
5MO9 - PubMed Abstract:
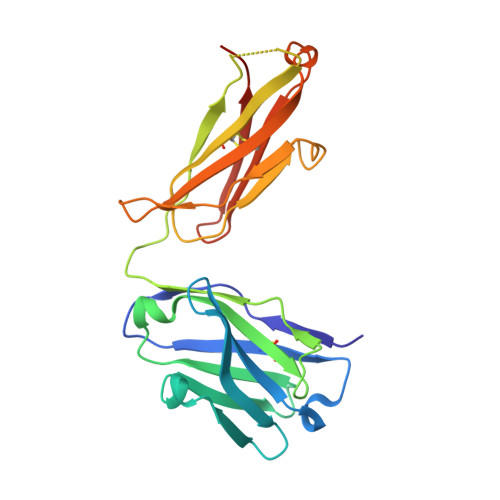
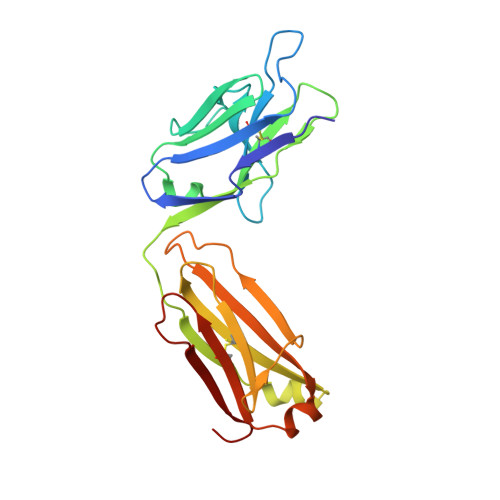
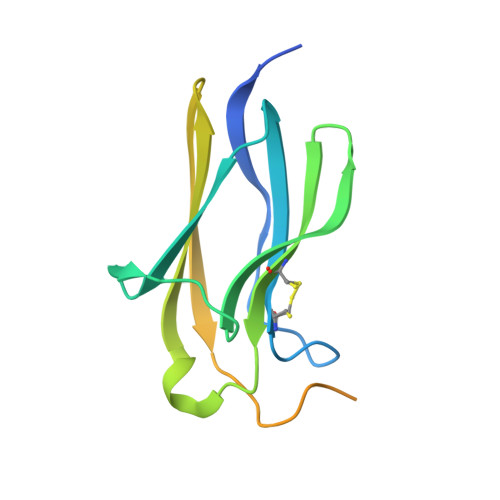
Brain-derived neurotrophic factor (BDNF) is a central modulator of neuronal development and synaptic plasticity in the central nervous system. This renders the BDNF-modulated tropomyosin receptor kinase B (TrkB) a promising drug target to treat synaptic dysfunctions. Using GR owth factor-driven expansion and IN hibition of N ot CH (GRINCH) during maturation, the so-called GRINCH neurons were derived from human-induced pluripotent stem cells. These GRINCH neurons were used as model cells for pharmacologic profiling of two TrkB-agonistic antibodies, hereafter referred to as AB2 and AB20 In next-generation sequencing studies, AB2 and AB20 stimulated transcriptional changes, which extensively overlapped with BDNF-driven transcriptional modulation. In regard to TrkB phosphorylation, both AB2 and AB20 were only about half as efficacious as BDNF; however, with respect to the TrkB downstream signaling, AB2 and AB20 displayed increased efficacy values, providing a stimulation at least comparable to BDNF in respect to VGF transcription, as well as of AKT and cAMP response element-binding protein phosphorylation. In a complex structure of the TrkB-d5 domain with AB20, determined by X-ray crystallography, the AB20 binding site was found to be allosteric in regard to the BDNF binding site, whereas AB2 was known to act orthosterically with BDNF. In agreement with this finding, AB2 and AB20 acted synergistically at greater concentrations to drive TrkB phosphorylation. Although TrkB downstream signaling declined faster after pulse stimulation with AB20 than with AB2, AB20 restimulated TrkB phosphorylation more efficiently than AB2. In conclusion, both antibodies displayed some limitations and some benefits in regard to future applications as therapeutic agents.
- Trenzyme GmbH, Konstanz (S.T.) Germany, Lead Identification and Optimization Support (L.H., S.H., R.H.), Immunological and Respiratory Diseases Research (H.S.), CNS Diseases Research (H.R.), and Target Discovery Research (E.S.), Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach, Germany; and Biotherapeutics Discovery, Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, Connecticut (L.F.).
Organizational Affiliation: