Structural Basis of Mec1-Ddc2-RPA Assembly and Activation on Single-Stranded DNA at Sites of Damage.
Deshpande, I., Seeber, A., Shimada, K., Keusch, J.J., Gut, H., Gasser, S.M.(2017) Mol Cell 68: 431-445.e5
- PubMed: 29033322
- DOI: https://doi.org/10.1016/j.molcel.2017.09.019
- Primary Citation of Related Structures:
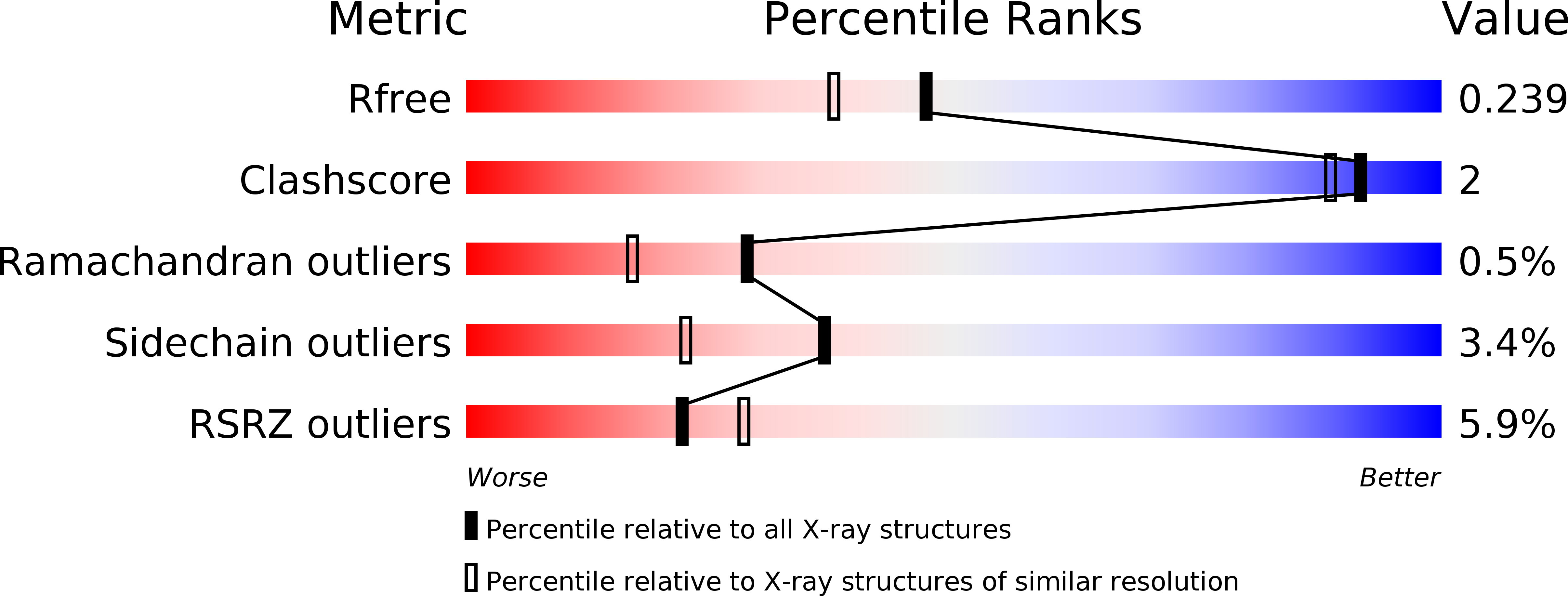
5OMB, 5OMC, 5OMD - PubMed Abstract:
Mec1-Ddc2 (ATR-ATRIP) is a key DNA-damage-sensing kinase that is recruited through the single-stranded (ss) DNA-binding replication protein A (RPA) to initiate the DNA damage checkpoint response. Activation of ATR-ATRIP in the absence of DNA damage is lethal. Therefore, it is important that damage-specific recruitment precedes kinase activation, which is achieved at least in part by Mec1-Ddc2 homodimerization. Here, we report a structural, biochemical, and functional characterization of the yeast Mec1-Ddc2-RPA assembly. High-resolution co-crystal structures of Ddc2-Rfa1 and Ddc2-Rfa1-t11 (K45E mutant) N termini and of the Ddc2 coiled-coil domain (CCD) provide insight into Mec1-Ddc2 homodimerization and damage-site targeting. Based on our structural and functional findings, we present a Mec1-Ddc2-RPA-ssDNA composite structural model. By way of validation, we show that RPA-dependent recruitment of Mec1-Ddc2 is crucial for maintaining its homodimeric state at ssDNA and that Ddc2's recruitment domain and CCD are important for Mec1-dependent survival of UV-light-induced DNA damage.
Organizational Affiliation:
Friedrich Miescher Institute for Biomedical Research (FMI), Maulbeerstrasse 66, 4058 Basel, Switzerland; University of Basel, Faculty of Natural Sciences, Klingelbergstrasse 50, 4056 Basel, Switzerland.