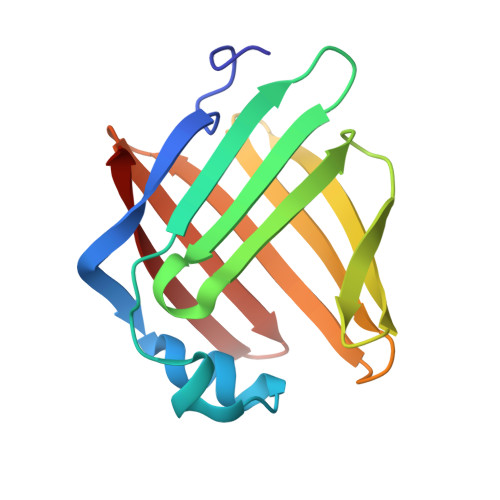
Structural insights into a secretory abundant heat-soluble protein from an anhydrobiotic tardigrade, Ramazzottius varieornatus
Fukuda, Y., Miura, Y., Mizohata, E., Inoue, T.(2017) FEBS Lett 591: 2458-2469
- PubMed: 28703282
- DOI: https://doi.org/10.1002/1873-3468.12752
- Primary Citation of Related Structures:
5XN9, 5XNA - PubMed Abstract:
Upon stopping metabolic processes, some tardigrades can undergo anhydrobiosis. Secretory abundant heat-soluble (SAHS) proteins have been reported as candidates for anhydrobiosis-related proteins in tardigrades, which seem to protect extracellular components and/or secretory organelles. We determined structures of a SAHS protein from Ramazzottius varieornatus (RvSAHS1), which is one of the toughest tardigrades. RvSAHS1 shows a β-barrel structure similar to fatty acid-binding proteins (FABPs), in which hydrophilic residues form peculiar hydrogen bond networks, which would provide RvSAHS1 with better tolerance against dehydration. We identified two putative ligand-binding sites: one that superimposes on those of some FABPs and the other, unique to and conserved in SAHS proteins. These results indicate that SAHS proteins constitute a new FABP family.
- Department of Applied Chemistry, Graduate School of Engineering, Osaka University, Suita, Osaka, Japan.
Organizational Affiliation: