Combined Biophysical Chemistry Reveals a New Covalent Inhibitor with a Low-Reactivity Alkyl Halide.
Li, T., Maltais, R., Poirier, D., Lin, S.X.(2018) J Phys Chem Lett 9: 5275-5280
- PubMed: 30148957
- DOI: https://doi.org/10.1021/acs.jpclett.8b02225
- Primary Citation of Related Structures:
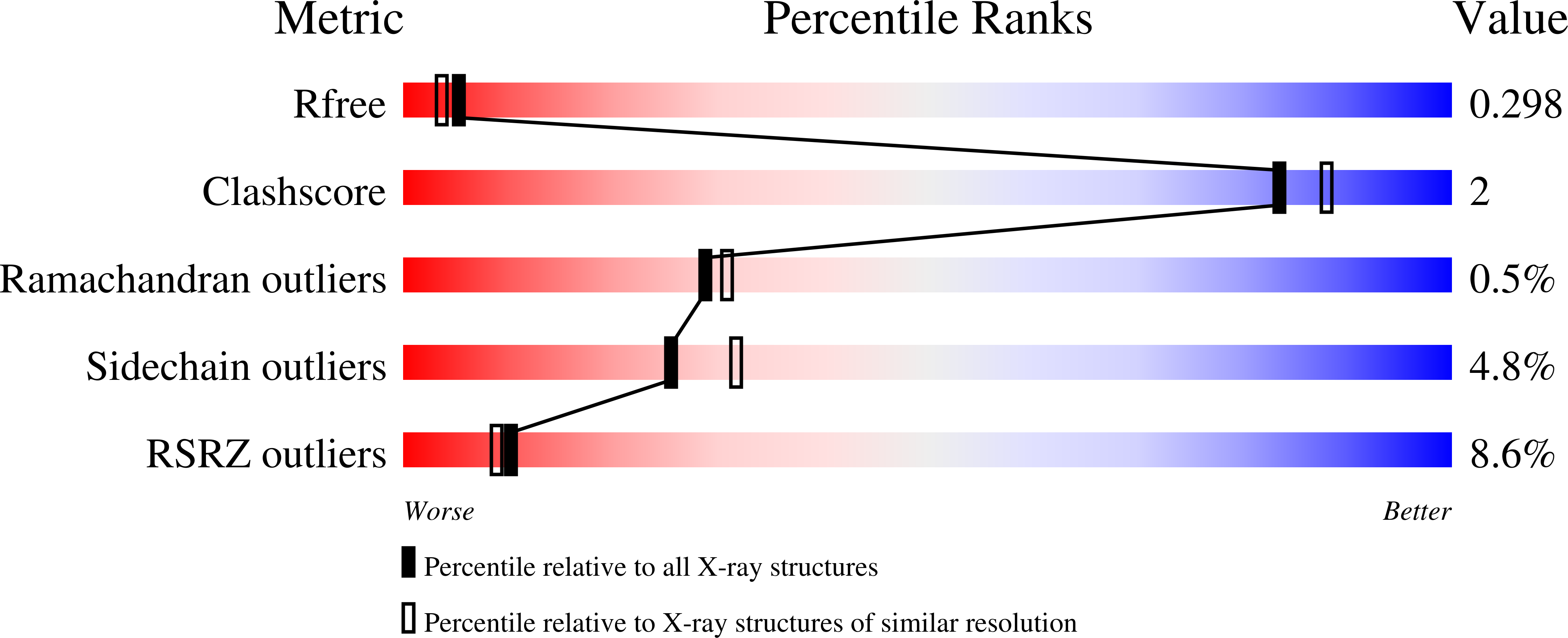
6CGC, 6CGE - PubMed Abstract:
17β-Hydroxysteroid dehydrogenase type 1 (17β-HSD1) plays a pivotal role in the progression of estrogen-related diseases because of its involvement in the biosynthesis of estradiol (E2), constituting a valuable therapeutic target for endocrine treatment. In the present study, we successfully cocrystallized the enzyme with the reversible inhibitor 2-methoxy-16β-( m-carbamoylbenzyl)-E2 (2-MeO-CC-156) as well as the enzyme with the irreversible inhibitor 3-(2-bromoethyl)-16β-( m-carbamoylbenzyl)-17β-hydroxy-1,3,5(10)-estratriene (PBRM). The structures of ternary complexes of 17β-HSD1-2-MeO-CC-156-NADP + and 17β-HSD1-PBRM-NADP + comparatively show the formation of a covalent bond between His 221 and the bromoethyl side chain of the inhibitor in the PBRM structure. A dynamic process including beneficial molecular interactions that favor the specific binding of a low-reactivity inhibitor and subsequent N-alkylation event through the participation of His 221 in the enzyme catalytic site clearly demonstrates the covalent bond formation. This finding opens the door to a new design of alkyl halide-based specific covalent inhibitors as potential therapeutic agents for different enzymes, contributing to the development of highly efficient inhibitors.
Organizational Affiliation:
CHU de Québec - Research Center , 2705 Boulevard Laurier , Québec , QC G1V 4G2 , Canada.