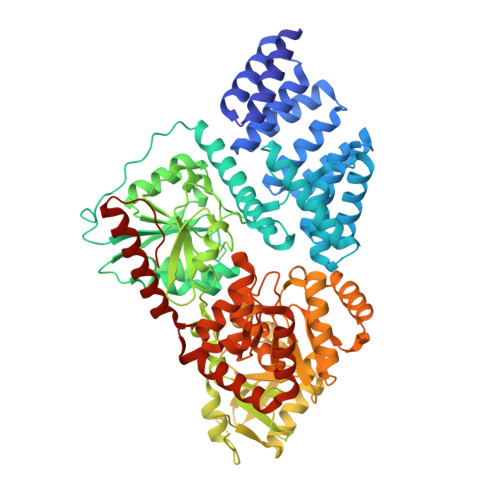
Targeted covalent inhibition of O-GlcNAc transferase in cells.
Worth, M., Hu, C.W., Li, H., Fan, D., Estevez, A., Zhu, D., Wang, A., Jiang, J.(2019) Chem Commun (Camb) 55: 13291-13294
- PubMed: 31626249
- DOI: https://doi.org/10.1039/c9cc04560k
- Primary Citation of Related Structures:
6E37 - PubMed Abstract:
O-GlcNAc transferase (OGT) glycosylates numerous proteins and is implicated in many diseases. To date, most OGT inhibitors lack either sufficient potency or characterized specificity in cells. We report the first targeted covalent inhibitor that predominantly reacts with OGT but does not affect other functionally similar enzymes. This study provides a new strategy to interrogate cellular OGT functions and to investigate other glycosyltransferases.
- Department of Chemistry, University of Wisconsin-Madison, Madison, Wisconsin 53705, USA.
Organizational Affiliation: