Preclinical Efficacy of Covalent-Allosteric AKT Inhibitor Borussertib in Combination with Trametinib inKRAS-Mutant Pancreatic and Colorectal Cancer.
Weisner, J., Landel, I., Reintjes, C., Uhlenbrock, N., Trajkovic-Arsic, M., Dienstbier, N., Hardick, J., Ladigan, S., Lindemann, M., Smith, S., Quambusch, L., Scheinpflug, R., Depta, L., Gontla, R., Unger, A., Muller, H., Baumann, M., Schultz-Fademrecht, C., Gunther, G., Maghnouj, A., Muller, M.P., Pohl, M., Teschendorf, C., Wolters, H., Viebahn, R., Tannapfel, A., Uhl, W., Hengstler, J.G., Hahn, S.A., Siveke, J.T., Rauh, D.(2019) Cancer Res 79: 2367-2378
- PubMed: 30858154
- DOI: https://doi.org/10.1158/0008-5472.CAN-18-2861
- Primary Citation of Related Structures:
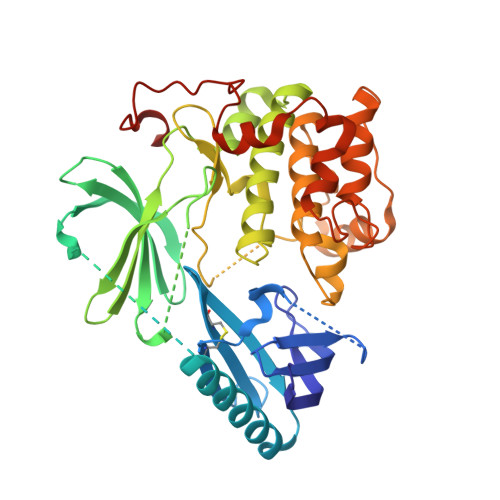
6HHF - PubMed Abstract:
Aberrations within the PI3K/AKT signaling axis are frequently observed in numerous cancer types, highlighting the relevance of these pathways in cancer physiology and pathology. However, therapeutic interventions employing AKT inhibitors often suffer from limitations associated with target selectivity, efficacy, or dose-limiting effects. Here we present the first crystal structure of autoinhibited AKT1 in complex with the covalent-allosteric inhibitor borussertib, providing critical insights into the structural basis of AKT1 inhibition by this unique class of compounds. Comprehensive biological and preclinical evaluation of borussertib in cancer-related model systems demonstrated a strong antiproliferative activity in cancer cell lines harboring genetic alterations within the PTEN, PI3K, and RAS signaling pathways. Furthermore, borussertib displayed antitumor activity in combination with the MEK inhibitor trametinib in patient-derived xenograft models of mutant KRAS pancreatic and colon cancer. SIGNIFICANCE: Borussertib, a first-in-class covalent-allosteric AKT inhibitor, displays antitumor activity in combination with the MEK inhibitor trametinib in patient-derived xenograft models and provides a starting point for further pharmacokinetic/dynamic optimization.
- Faculty of Chemistry and Chemical Biology, TU Dortmund University, Dortmund, Germany.
Organizational Affiliation: