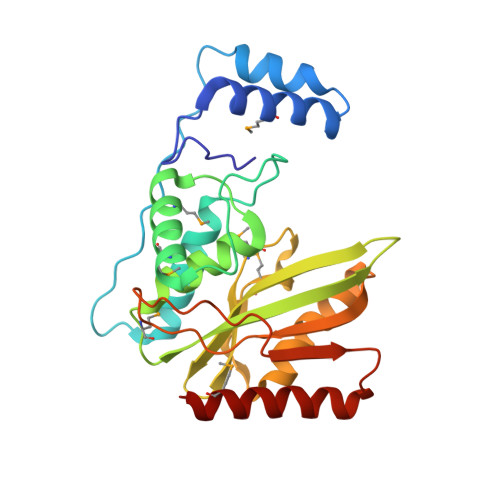
Structural basis of tubulin detyrosination by vasohibins.
Li, F., Hu, Y., Qi, S., Luo, X., Yu, H.(2019) Nat Struct Mol Biol 26: 583-591
- PubMed: 31235910
- DOI: https://doi.org/10.1038/s41594-019-0242-x
- Primary Citation of Related Structures:
6OCF, 6OCG, 6OCH - PubMed Abstract:
Microtubules are regulated by post-translational modifications of tubulin. The ligation and cleavage of the carboxy-terminal tyrosine of α-tubulin impact microtubule functions during mitosis, cardiomyocyte contraction and neuronal processes. Tubulin tyrosination and detyrosination are mediated by tubulin tyrosine ligase and the recently discovered tubulin detyrosinases, vasohibin 1 and 2 (VASH1 and VASH2) bound to the small vasohibin-binding protein (SVBP). Here, we report the crystal structures of human VASH1-SVBP alone, in complex with a tyrosine-derived covalent inhibitor and bound to the natural product parthenolide. The structures and subsequent mutagenesis analyses explain the requirement for SVBP during tubulin detyrosination, and reveal the basis for the recognition of the C-terminal tyrosine and the acidic α-tubulin tail by VASH1. The VASH1-SVBP-parthenolide structure provides a framework for designing more effective chemical inhibitors of vasohibins, which can be valuable for dissecting their biological functions and may have therapeutic potential.
- Department of Pharmacology, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Organizational Affiliation: