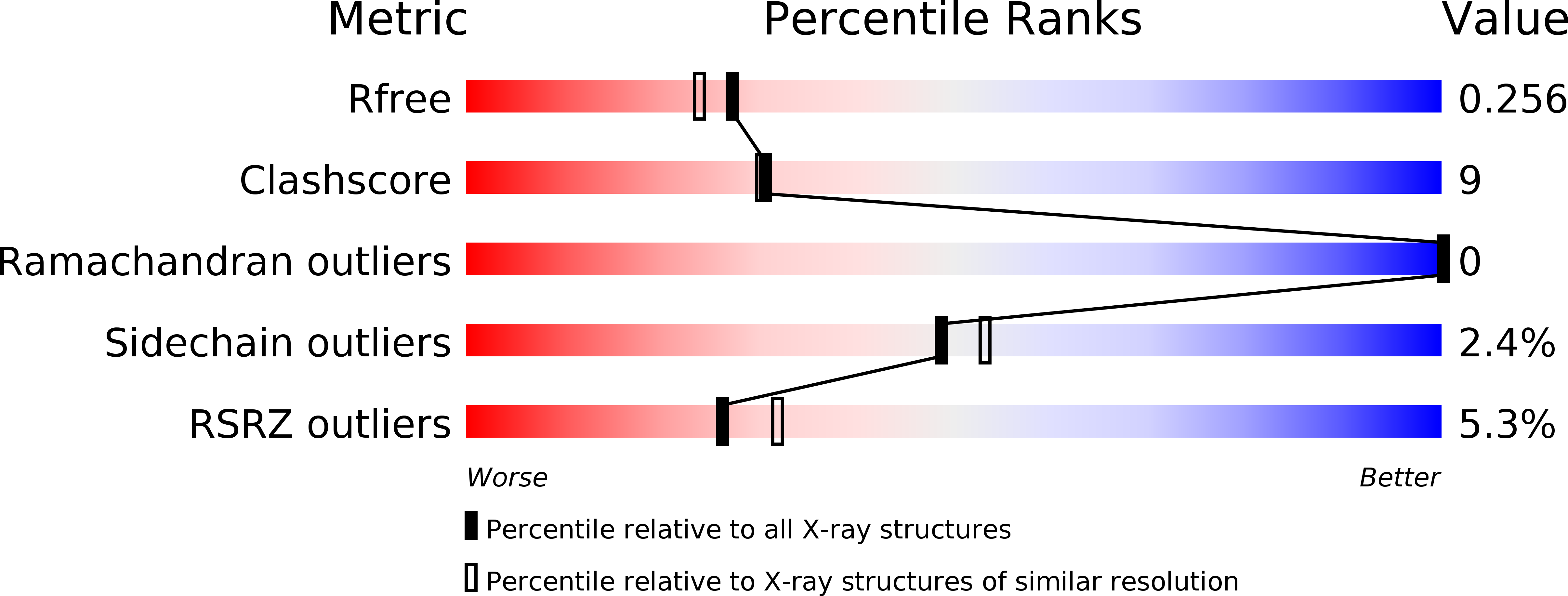
Molecular Basis for Spirocycle Formation in the Paraherquamide Biosynthetic Pathway.
Fraley, A.E., Caddell Haatveit, K., Ye, Y., Kelly, S.P., Newmister, S.A., Yu, F., Williams, R.M., Smith, J.L., Houk, K.N., Sherman, D.H.(2020) J Am Chem Soc 142: 2244-2252
- PubMed: 31904957
- DOI: https://doi.org/10.1021/jacs.9b09070
- Primary Citation of Related Structures:
6PVF, 6PVG, 6PVH, 6PVI, 6PVJ - PubMed Abstract:
The paraherquamides are potent anthelmintic natural products with complex heptacyclic scaffolds. One key feature of these molecules is the spiro-oxindole moiety that lends a strained three-dimensional architecture to these structures. The flavin monooxygenase PhqK was found to catalyze spirocycle formation through two parallel pathways in the biosynthesis of paraherquamides A and G. Two new paraherquamides (K and L) were isolated from a Δ phqK strain of Penicillium simplicissimum , and subsequent enzymatic reactions with these compounds generated two additional metabolites, paraherquamides M and N. Crystal structures of PhqK in complex with various substrates provided a foundation for mechanistic analyses and computational studies. While it is evident that PhqK can react with various substrates, reaction kinetics and molecular dynamics simulations indicated that the dioxepin-containing paraherquamide L is the favored substrate. Through this effort, we have elucidated a key step in the biosynthesis of the paraherquamides and provided a rationale for the selective spirocyclization of these powerful anthelmintic agents.
Organizational Affiliation:
Department of Chemistry and Biochemistry , University of California , Los Angeles , California 90095 , United States.