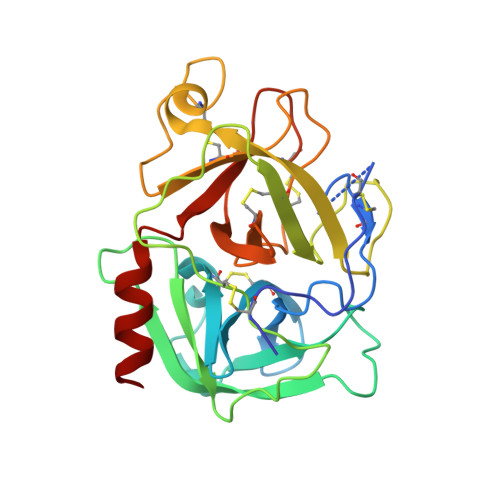
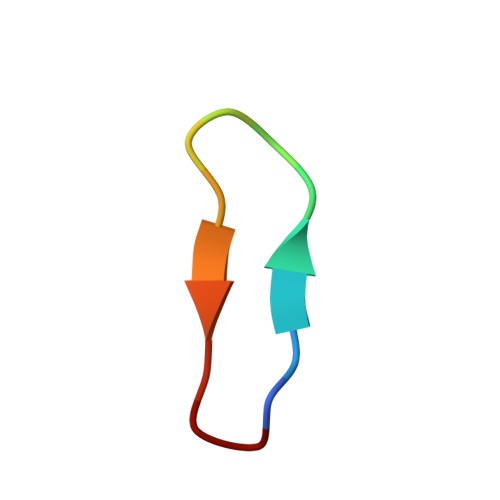
Application and Structural Analysis of Triazole-Bridged Disulfide Mimetics in Cyclic Peptides.
White, A.M., de Veer, S.J., Wu, G., Harvey, P.J., Yap, K., King, G.J., Swedberg, J.E., Wang, C.K., Law, R.H.P., Durek, T., Craik, D.J.(2020) Angew Chem Int Ed Engl 59: 11273-11277
- PubMed: 32270580
- DOI: https://doi.org/10.1002/anie.202003435
- Primary Citation of Related Structures:
6Q1U, 6U22, 6U24, 6U7Q, 6U7R, 6U7S, 6U7U, 6U7W, 6U7X, 6VXY, 6VY8 - PubMed Abstract:
Ruthenium-catalysed azide-alkyne cycloaddition (RuAAC) provides access to 1,5-disubstituted 1,2,3-triazole motifs in peptide engineering applications. However, investigation of this motif as a disulfide mimetic in cyclic peptides has been limited, and the structural consequences remain to be studied. We report synthetic strategies to install various triazole linkages into cyclic peptides through backbone cyclisation and RuAAC cross-linking reactions. These linkages were evaluated in four serine protease inhibitors based on sunflower trypsin inhibitor-1. NMR and X-ray crystallography revealed exceptional consensus of bridging distance and backbone conformations (RMSD<0.5 Å) of the triazole linkages compared to the parent disulfide molecules. The triazole-bridged peptides also displayed superior half-lives in liver S9 stability assays compared to disulfide-bridged peptides. This work establishes a foundation for the application of 1,5-disubstituted 1,2,3-triazoles as disulfide mimetics.
- ARC Centre of Excellence for Innovations in Peptide and Protein Science, Institute for Molecular Bioscience, The University of Queensland, Brisbane, QLD, 4072, Australia.
Organizational Affiliation: