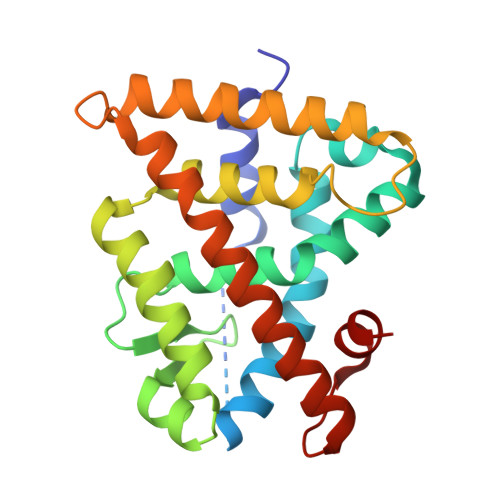
A Novel Biphenyl-based Chemotype of Retinoid X Receptor Ligands Enables Subtype and Heterodimer Preferences.
Pollinger, J., Schierle, S., Gellrich, L., Ohrndorf, J., Kaiser, A., Heitel, P., Chaikuad, A., Knapp, S., Merk, D.(2019) ACS Med Chem Lett 10: 1346-1352
- PubMed: 31531208
- DOI: https://doi.org/10.1021/acsmedchemlett.9b00306
- Primary Citation of Related Structures:
6SJM - PubMed Abstract:
The nuclear retinoid X receptors (RXRs) are key ligand sensing transcription factors that serve as universal nuclear receptor heterodimer partners and are thus involved in numerous physiological processes. Therapeutic targeting of RXRs holds high potential but available RXR activators suffer from limited safety. Selectivity for RXR subtypes or for certain RXR heterodimers are promising strategies for safer RXR modulation. Here, we report systematic structure-activity relationship studies on biphenyl carboxylates as new RXR ligand chemotype. We discovered specific structural modifications that enhance potency on RXRs, govern subtype preference, and vary modulation of different RXR heterodimers. Fusion of these structural motifs enabled specific tuning of subtype preferential profiles with markedly improved potency. Our results provide further evidence that RXR subtype selective ligands can be designed and present a novel chemotype of RXR modulators that can be tuned for subtype and heterodimer preferences.
- Institute of Pharmaceutical Chemistry, Goethe University Frankfurt, Max-von-Laue-Str. 9, D-60438 Frankfurt, Germany.
Organizational Affiliation: