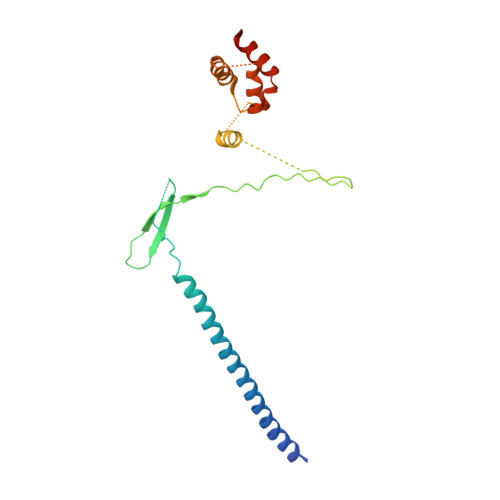
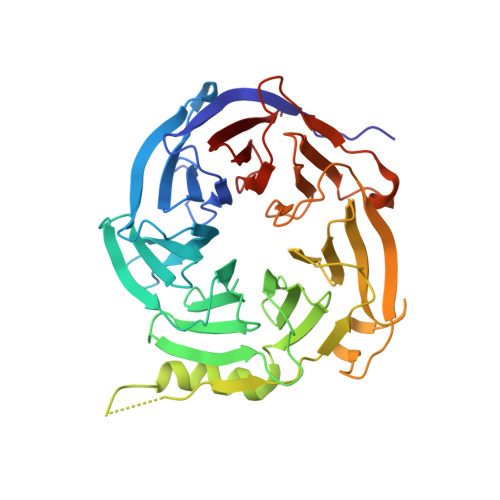
A partially disordered region connects gene repression and activation functions of EZH2.
Jiao, L., Shubbar, M., Yang, X., Zhang, Q., Chen, S., Wu, Q., Chen, Z., Rizo, J., Liu, X.(2020) Proc Natl Acad Sci U S A 117: 16992-17002
- PubMed: 32631994
- DOI: https://doi.org/10.1073/pnas.1914866117
- Primary Citation of Related Structures:
6U4Y - PubMed Abstract:
Enhancer of Zeste Homolog 2 (EZH2) is the catalytic subunit of Polycomb Repressive Complex 2 (PRC2), which minimally requires two other subunits, EED and SUZ12, for enzymatic activity. EZH2 has been traditionally known to mediate histone H3K27 trimethylation, a hallmark of silent chromatin. Emerging evidence indicates that EZH2 also activates gene expression in cancer cells in a context distinct from canonical PRC2. The molecular mechanism underlying the functional conversion of EZH2 from a gene repressor to an activator is unclear. Here, we show that EZH2 harbors a hidden, partially disordered transactivation domain (TAD) capable of interacting with components of active transcription machinery, mimicking archetypal acidic activators. The EZH2 TAD comprises the SRM (Stimulation-Responsive Motif) and SANT1 (SWI3, ADA2, N-CoR, and TFIIIB 1) regions that are normally involved in H3K27 methylation. The crystal structure of an EZH2-EED binary complex indicates that the EZH2 TAD mediates protein oligomerization in a noncanonical PRC2 context and is entirely sequestered. The EZH2 TAD can be unlocked by cancer-specific EZH2 phosphorylation events to undergo structural transitions that may enable subsequent transcriptional coactivator binding. The EZH2 TAD directly interacts with the transcriptional coactivator and histone acetyltransferase p300 and activates gene expression in a p300-dependent manner in cells. The corresponding TAD may also account for the gene activation function of EZH1, the paralog of EZH2. Distinct kinase signaling pathways that are known to abnormally convert EZH2 into a gene activator in cancer cells can now be understood in a common structural context of the EZH2 TAD.
- Cecil H. and Ida Green Center for Reproductive Biology Sciences, University of Texas Southwestern Medical Center, Dallas, TX 75390.
Organizational Affiliation: