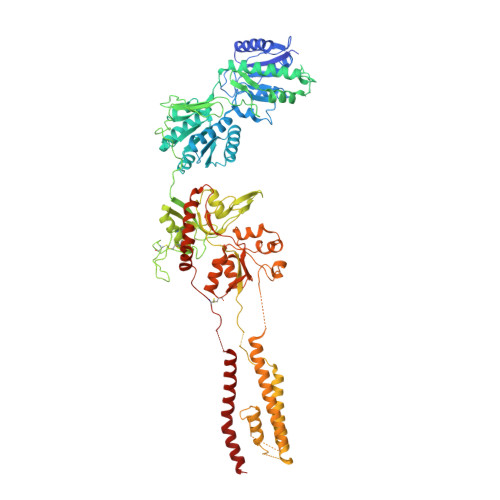
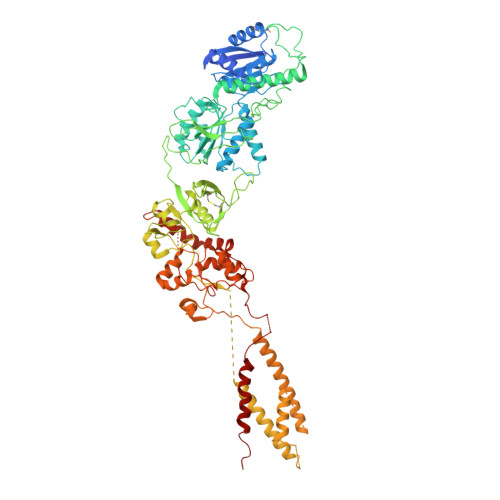
Structural Basis of the Proton Sensitivity of Human GluN1-GluN2A NMDA Receptors
Zhang, J.B., Chang, S., Xu, P., Miao, M., Wu, H., Zhang, Y., Zhang, T., Wang, H., Zhang, J., Xie, C., Song, N., Luo, C., Zhang, X., Zhu, S.(2018) Cell Rep 25: 3582-3590.e4
- PubMed: 30590034
- DOI: https://doi.org/10.1016/j.celrep.2018.11.071
- Primary Citation of Related Structures:
6IRA, 6IRF, 6IRG, 6IRH - PubMed Abstract:
N-methyl-D-aspartate (NMDA) receptors are critical for synaptic development and plasticity. While glutamate is the primary agonist, protons can modulate NMDA receptor activity at synapses during vesicle exocytosis by mechanisms that are unknown. We used cryo-electron microscopy to solve the structures of the human GluN1-GluN2A NMDA receptor at pH 7.8 and pH 6.3. Our structures demonstrate that the proton sensor predominantly resides in the N-terminal domain (NTD) of the GluN2A subunit and reveal the allosteric coupling mechanism between the proton sensor and the channel gate. Under high-pH conditions, the GluN2A-NTD adopts an "open-and-twisted" conformation. However, upon protonation at the lower pH, the GluN2A-NTD transits from an open- to closed-cleft conformation, causing rearrangements between the tetrameric NTDs and agonist-binding domains. The conformational mobility observed in our structures (presumably from protonation) is supported by molecular dynamics simulation. Our findings reveal the structural mechanisms by which protons allosterically inhibit human GluN1-GluN2A receptor activity.
- Institute of Neuroscience, CAS Center for Excellence in Brain Science and Intelligence Technology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai 200031, China.
Organizational Affiliation: