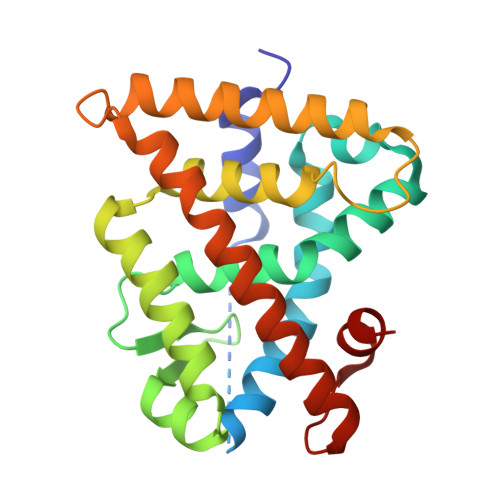
Oxaprozin Analogues as Selective RXR Agonists with Superior Properties and Pharmacokinetics.
Schierle, S., Chaikuad, A., Lillich, F.F., Ni, X., Woltersdorf, S., Schallmayer, E., Renelt, B., Ronchetti, R., Knapp, S., Proschak, E., Merk, D.(2021) J Med Chem 64: 5123-5136
- PubMed: 33793232
- DOI: https://doi.org/10.1021/acs.jmedchem.1c00235
- Primary Citation of Related Structures:
7B88, 7B9O - PubMed Abstract:
The retinoid X receptors (RXR) are ligand-activated transcription factors involved in multiple regulatory networks as universal heterodimer partners for nuclear receptors. Despite their high therapeutic potential in many pathologies, targeting of RXR has only been exploited in cancer treatment as the currently available RXR agonists suffer from exceptional lipophilicity, poor pharmacokinetics (PK), and adverse effects. Aiming to overcome the limitations and to provide improved RXR ligands, we developed a new potent RXR ligand chemotype based on the nonsteroidal anti-inflammatory drug oxaprozin. Systematic structure-activity relationship analysis enabled structural optimization toward low nanomolar potency similar to the well-established rexinoids. Cocrystal structures of the most active derivatives demonstrated orthosteric binding, and in vivo profiling revealed superior PK properties compared to current RXR agonists. The optimized compounds were highly selective for RXR activation and induced RXR-regulated gene expression in native cellular and in vivo settings suggesting them as excellent chemical tools to further explore the therapeutic potential of RXR.
- Institute of Pharmaceutical Chemistry, Goethe University Frankfurt, Max-von-Laue-Str. 9, D-60438 Frankfurt, Germany.
Organizational Affiliation: