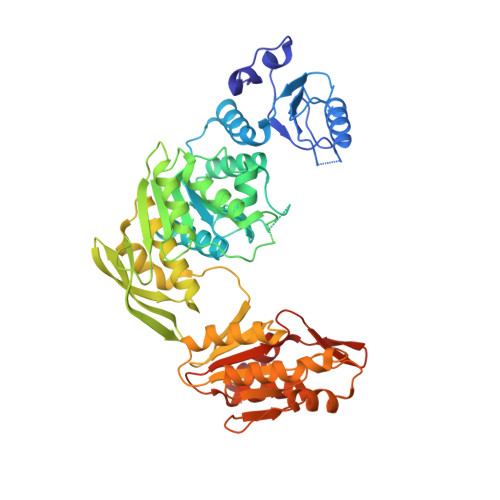
Wide-open conformation of UDP-MurNc-tripeptide ligase revealed by the substrate-free structure of MurE from Acinetobacter baumannii.
Jung, K.H., Kim, Y.G., Kim, C.M., Ha, H.J., Lee, C.S., Lee, J.H., Park, H.H.(2021) FEBS Lett 595: 275-283
- PubMed: 33230844
- DOI: https://doi.org/10.1002/1873-3468.14007
- Primary Citation of Related Structures:
7D27 - PubMed Abstract:
MurE ligase catalyzes the attachment of meso-diaminopimelic acid to the UDP-MurNAc- l -Ala- d -Glu using ATP and producing UDP-MurNAc- l -Ala- d -Glu-meso-A 2 pm during bacterial cell wall biosynthesis. Owing to the critical role of this enzyme, MurE is considered an attractive target for antibacterial drugs. Despite extensive studies on MurE ligase, the structural dynamics of its conformational changes are still elusive. In this study, we present the substrate-free structure of MurE from Acinetobacter baumannii, which is an antibiotic-resistant superbacterium that has threatened global public health. The structure revealed that MurE has a wide-open conformation and undergoes wide-open, intermediately closed, and fully closed dynamic conformational transition. Unveiling structural dynamics of MurE will help to understand the working mechanism of this ligase and to design next-generation antibiotics targeting MurE.
Organizational Affiliation:
Department of Global Innovative Drugs, Graduate School of Chung-Ang University, Seoul, Korea.