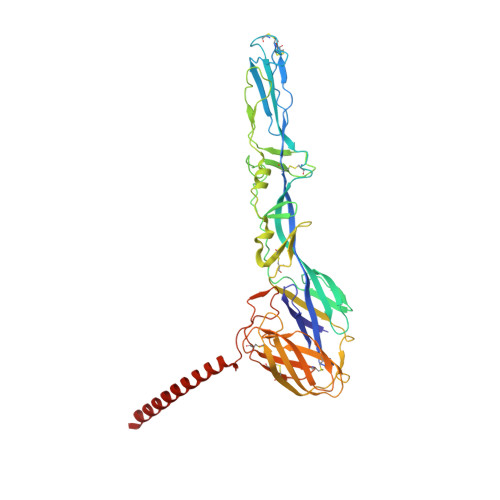
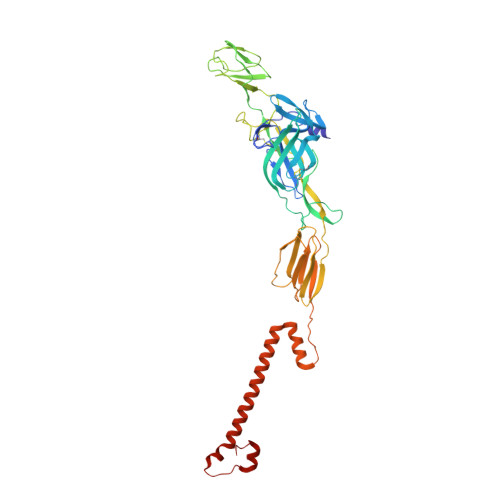
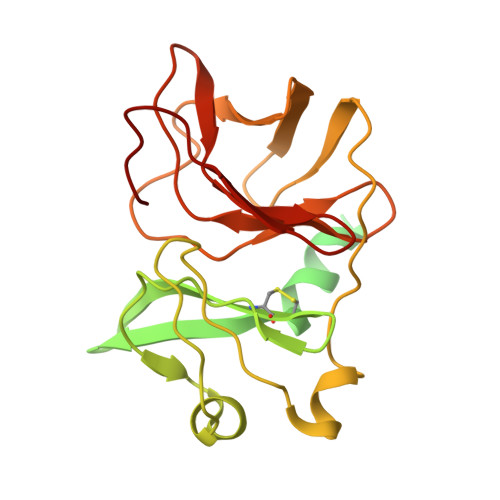
Structure of infective Getah virus at 2.8 angstrom resolution determined by cryo-electron microscopy.
Wang, A., Zhou, F., Liu, C., Gao, D., Qi, R., Yin, Y., Liu, S., Gao, Y., Fu, L., Xia, Y., Xu, Y., Wang, C., Liu, Z.(2022) Cell Discov 8: 12-12
- PubMed: 35149682
- DOI: https://doi.org/10.1038/s41421-022-00374-6
- Primary Citation of Related Structures:
7FD2 - PubMed Abstract:
Getah virus (GETV), a member of the genus alphavirus, is a mosquito-borne pathogen that can cause pyrexia and reproductive losses in animals. Although antibodies to GETV have been found in over 10% of healthy people, there are no reports of clinical symptoms associated with GETV. The biological and pathological properties of GETV are largely unknown and antiviral or vaccine treatments against GETV are still unavailable due to a lack of knowledge of the structure of the GETV virion. Here, we present the structure of infective GETV at a resolution of 2.8 Å with the atomic models of the capsid protein and the envelope glycoproteins E1 and E2. We have identified numerous glycosylation and S-acylation sites in E1 and E2. The surface-exposed glycans indicate a possible impact on viral immune evasion and host cell invasion. The S-acylation sites might be involved in stabilizing the transmembrane assembly of E1 and E2. In addition, a cholesterol and a phospholipid molecule are observed in a transmembrane hydrophobic pocket, together with two more cholesterols surrounding the pocket. The cholesterol and phospholipid stabilize the hydrophobic pocket in the viral envelope membrane. The structural information will assist structure-based antiviral and vaccine screening, design, and optimization.
Organizational Affiliation:
Cryo-electron Microscopy Center, Southern University of Science and Technology, Shenzhen, Guangdong, China.