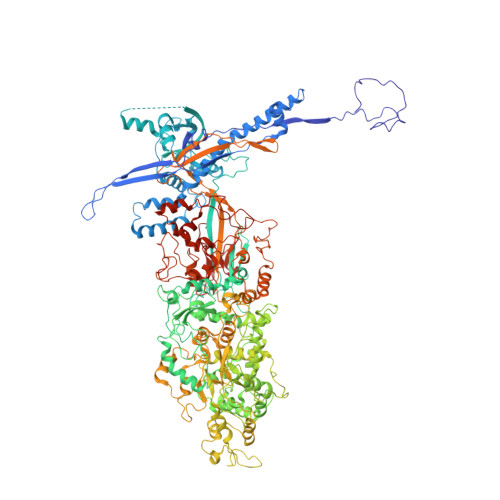
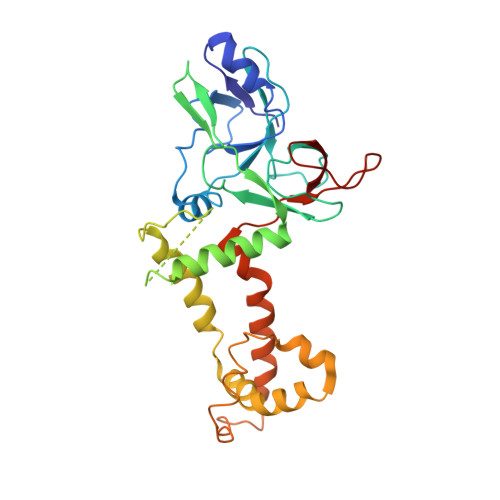
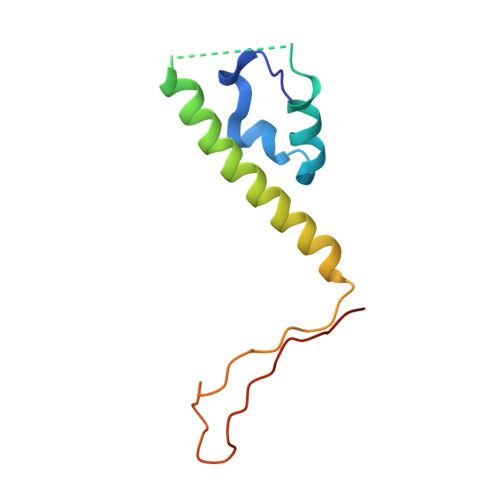
Structures of pseudorabies virus capsids.
Wang, G., Zha, Z., Huang, P., Sun, H., Huang, Y., He, M., Chen, T., Lin, L., Chen, Z., Kong, Z., Que, Y., Li, T., Gu, Y., Yu, H., Zhang, J., Zheng, Q., Chen, Y., Li, S., Xia, N.(2022) Nat Commun 13: 1533-1533
- PubMed: 35318331
- DOI: https://doi.org/10.1038/s41467-022-29250-3
- Primary Citation of Related Structures:
7FJ1, 7FJ3 - PubMed Abstract:
Pseudorabies virus (PRV) is a major etiological agent of swine infectious diseases and is responsible for significant economic losses in the swine industry. Recent data points to human viral encephalitis caused by PRV infection, suggesting that PRV may be able to overcome the species barrier to infect humans. To date, there is no available therapeutic for PRV infection. Here, we report the near-atomic structures of the PRV A-capsid and C-capsid, and illustrate the interaction that occurs between these subunits. We show that the C-capsid portal complex is decorated with capsid-associated tegument complexes. The PRV capsid structure is highly reminiscent of other α-herpesviruses, with some additional structural features of β- and γ-herpesviruses. These results illustrate the structure of the PRV capsid and elucidate the underlying assembly mechanism at the molecular level. This knowledge may be useful for the development of oncolytic agents or specific therapeutics against this arm of the herpesvirus family.
Organizational Affiliation:
State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, School of Public Health, School of Life Sciences, Xiamen University, Xiamen, 361102, China.