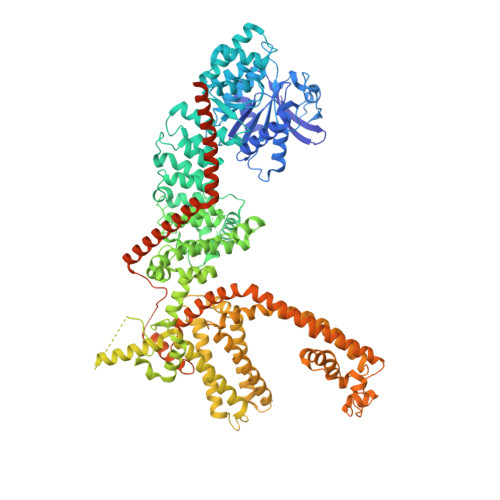
Structures of the TRPM5 channel elucidate mechanisms of activation and inhibition.
Ruan, Z., Haley, E., Orozco, I.J., Sabat, M., Myers, R., Roth, R., Du, J., Lu, W.(2021) Nat Struct Mol Biol 28: 604-613
- PubMed: 34168372
- DOI: https://doi.org/10.1038/s41594-021-00607-4
- Primary Citation of Related Structures:
7MBP, 7MBQ, 7MBR, 7MBS, 7MBT, 7MBU, 7MBV - PubMed Abstract:
The Ca 2+ -activated TRPM5 channel plays essential roles in taste perception and insulin secretion. However, the mechanism by which Ca 2+ regulates TRPM5 activity remains elusive. We report cryo-EM structures of the zebrafish TRPM5 in an apo closed state, a Ca 2+ -bound open state, and an antagonist-bound inhibited state. We define two novel ligand binding sites: a Ca 2+ site (Ca ICD ) in the intracellular domain and an antagonist site in the transmembrane domain (TMD). The Ca ICD site is unique to TRPM5 and has two roles: modulating the voltage dependence and promoting Ca 2+ binding to the Ca TMD site, which is conserved throughout TRPM channels. Conformational changes initialized from both Ca 2+ sites cooperatively open the ion-conducting pore. The antagonist NDNA wedges into the space between the S1-S4 domain and pore domain, stabilizing the transmembrane domain in an apo-like closed state. Our results lay the foundation for understanding the voltage-dependent TRPM channels and developing new therapeutic agents.
Organizational Affiliation:
Van Andel Institute, Grand Rapids, MI, USA.