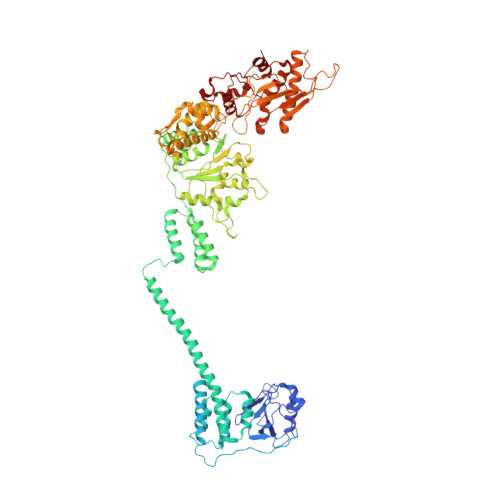
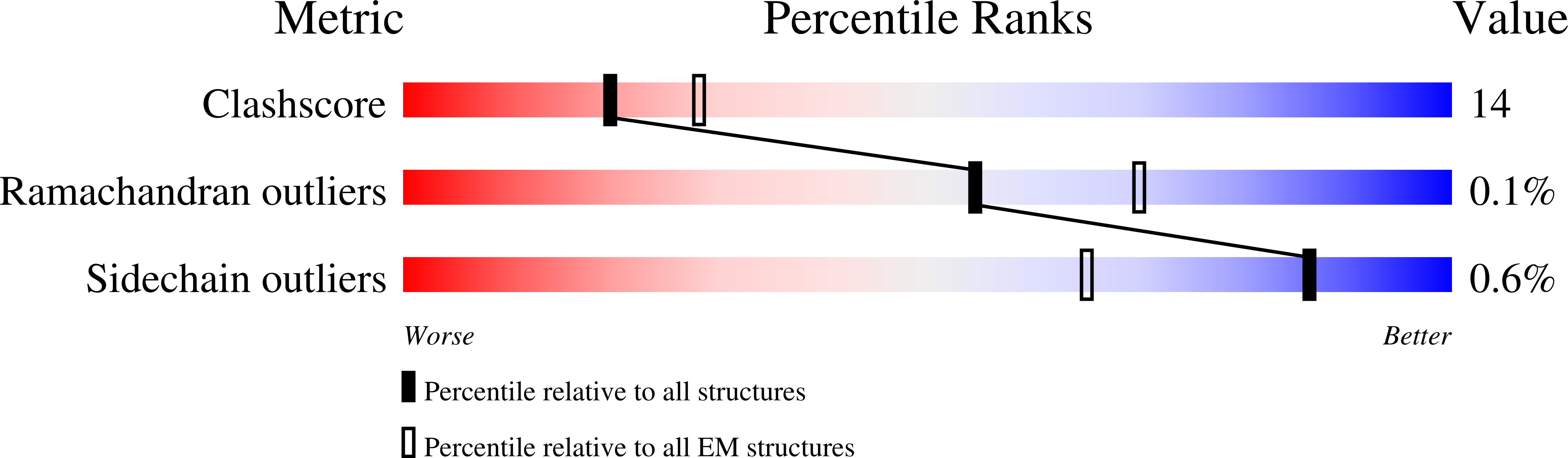Catalytic cycling of human mitochondrial Lon protease.
Mohammed, I., Schmitz, K.A., Schenck, N., Balasopoulos, D., Topitsch, A., Maier, T., Abrahams, J.P.(2022) Structure 30: 1254-1268.e7
- PubMed: 35870450
- DOI: https://doi.org/10.1016/j.str.2022.06.006
- Primary Citation of Related Structures:
7NFY, 7NG4, 7NG5, 7NGC, 7NGF, 7NGL, 7NGP, 7NGQ, 7OXO - PubMed Abstract:
The mitochondrial Lon protease (LonP1) regulates mitochondrial health by removing redundant proteins from the mitochondrial matrix. We determined LonP1 in eight nucleotide-dependent conformational states by cryoelectron microscopy (cryo-EM). The flexible assembly of N-terminal domains had 3-fold symmetry, and its orientation depended on the conformational state. We show that a conserved structural motif around T803 with a high similarity to the trypsin catalytic triad is essential for proteolysis. We show that LonP1 is not regulated by redox potential, despite the presence of two conserved cysteines at disulfide-bonding distance in its unfoldase core. Our data indicate how sequential ATP hydrolysis controls substrate protein translocation in a 6-fold binding change mechanism. Substrate protein translocation, rather than ATP hydrolysis, is a rate-limiting step, suggesting that LonP1 is a Brownian ratchet with ATP hydrolysis preventing translocation reversal. 3-fold rocking motions of the flexible N-domain assembly may assist thermal unfolding of the substrate protein.
Organizational Affiliation:
Biozentrum, University of Basel, Basel, Switzerland.