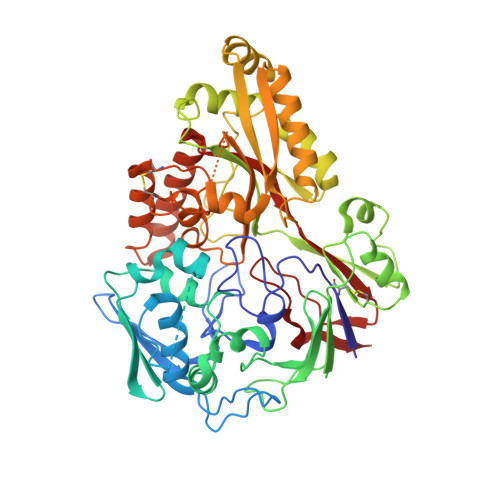
The structure of nontypeable Haemophilus influenzae SapA in a closed conformation reveals a constricted ligand-binding cavity and a novel RNA binding motif.
Lukacik, P., Owen, C.D., Harris, G., Bolla, J.R., Picaud, S., Alibay, I., Nettleship, J.E., Bird, L.E., Owens, R.J., Biggin, P.C., Filippakopoulos, P., Robinson, C.V., Walsh, M.A.(2021) PLoS One 16: e0256070-e0256070
- PubMed: 34653190
- DOI: https://doi.org/10.1371/journal.pone.0256070
- Primary Citation of Related Structures:
7OFW, 7OFZ, 7OG0 - PubMed Abstract:
Nontypeable Haemophilus influenzae (NTHi) is a significant pathogen in respiratory disease and otitis media. Important for NTHi survival, colonization and persistence in vivo is the Sap (sensitivity to antimicrobial peptides) ABC transporter system. Current models propose a direct role for Sap in heme and antimicrobial peptide (AMP) transport. Here, the crystal structure of SapA, the periplasmic component of Sap, in a closed, ligand bound conformation, is presented. Phylogenetic and cavity volume analysis predicts that the small, hydrophobic SapA central ligand binding cavity is most likely occupied by a hydrophobic di- or tri- peptide. The cavity is of insufficient volume to accommodate heme or folded AMPs. Crystal structures of SapA have identified surface interactions with heme and dsRNA. Heme binds SapA weakly (Kd 282 μM) through a surface exposed histidine, while the dsRNA is coordinated via residues which constitute part of a conserved motif (estimated Kd 4.4 μM). The RNA affinity falls within the range observed for characterized RNA/protein complexes. Overall, we describe in molecular-detail the interactions of SapA with heme and dsRNA and propose a role for SapA in the transport of di- or tri-peptides.
Organizational Affiliation:
Diamond Light Source, Harwell Science & Innovation Campus, Didcot, Oxfordshire, United Kingdom.