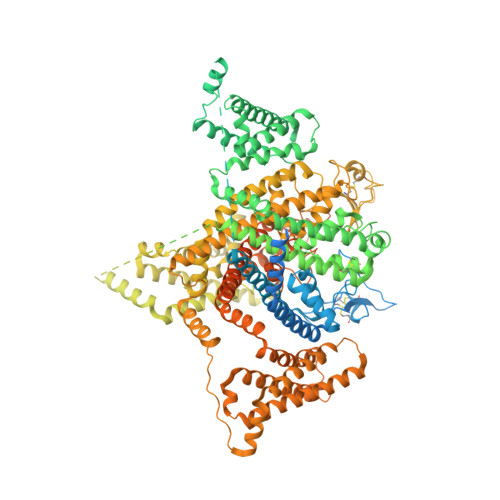
Structural basis for high-voltage activation and subtype-specific inhibition of human Na v 1.8.
Huang, X., Jin, X., Huang, G., Huang, J., Wu, T., Li, Z., Chen, J., Kong, F., Pan, X., Yan, N.(2022) Proc Natl Acad Sci U S A 119: e2208211119-e2208211119
- PubMed: 35858452
- DOI: https://doi.org/10.1073/pnas.2208211119
- Primary Citation of Related Structures:
7WFW - PubMed Abstract:
The dorsal root ganglia-localized voltage-gated sodium (Na v ) channel Na v 1.8 represents a promising target for developing next-generation analgesics. A prominent characteristic of Na v 1.8 is the requirement of more depolarized membrane potential for activation. Here we present the cryogenic electron microscopy structures of human Na v 1.8 alone and bound to a selective pore blocker, A-803467, at overall resolutions of 2.7 to 3.2 Å. The first voltage-sensing domain (VSD I ) displays three different conformations. Structure-guided mutagenesis identified the extracellular interface between VSD I and the pore domain (PD) to be a determinant for the high-voltage dependence of activation. A-803467 was clearly resolved in the central cavity of the PD, clenching S6 IV . Our structure-guided functional characterizations show that two nonligand binding residues, Thr397 on S6 I and Gly1406 on S6 III , allosterically modulate the channel's sensitivity to A-803467. Comparison of available structures of human Na v channels suggests the extracellular loop region to be a potential site for developing subtype-specific pore-blocking biologics.
Organizational Affiliation:
State Key Laboratory of Membrane Biology, Beijing Advanced Innovation Center for Structural Biology, Tsinghua-Peking Joint Center for Life Sciences, School of Life Sciences, Tsinghua University, Beijing 100084, China.