Structural basis for ERK2 allosteric inhibitors.
Yoshida, M., Kinoshita, T.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Mitogen-activated protein kinase 1 | 368 | Homo sapiens | Mutation(s): 0 Gene Names: MAPK1, ERK2, PRKM1, PRKM2 EC: 2.7.11.24 | 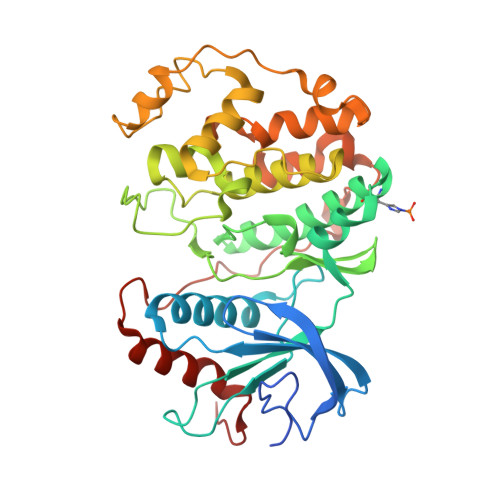 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P28482 (Homo sapiens) Explore P28482 Go to UniProtKB: P28482 | |||||
PHAROS: P28482 GTEx: ENSG00000100030 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P28482 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 5 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| B8Z Query on B8Z | G [auth A] | ~{N}-(5,6-dimethoxy-1,3-benzothiazol-2-yl)-2-[(4-fluoranylphenoxy)methyl]-1,3-thiazole-4-carboxamide C20 H16 F N3 O4 S2 PCKXVDUIUFNAJD-UHFFFAOYSA-N |  | ||
| 5ID Query on 5ID | C [auth A], J [auth B] | (2R,3R,4S,5R)-2-(4-AMINO-5-IODO-7H-PYRROLO[2,3-D]PYRIMIDIN-7-YL)-5-(HYDROXYMETHYL)TETRAHYDROFURAN-3,4-DIOL C11 H13 I N4 O4 WHSIXKUPQCKWBY-IOSLPCCCSA-N |  | ||
| EPE Query on EPE | D [auth A], I [auth B] | 4-(2-HYDROXYETHYL)-1-PIPERAZINE ETHANESULFONIC ACID C8 H18 N2 O4 S JKMHFZQWWAIEOD-UHFFFAOYSA-N |  | ||
| SO4 Query on SO4 | H [auth A] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| GOL Query on GOL | E [auth A], F [auth A], K [auth B], L [auth B] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| NEP Query on NEP | A, B | L-PEPTIDE LINKING | C6 H10 N3 O5 P |  | HIS |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 82.653 | α = 90 |
| b = 82.653 | β = 90 |
| c = 275.074 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| XDS | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Not funded | -- |