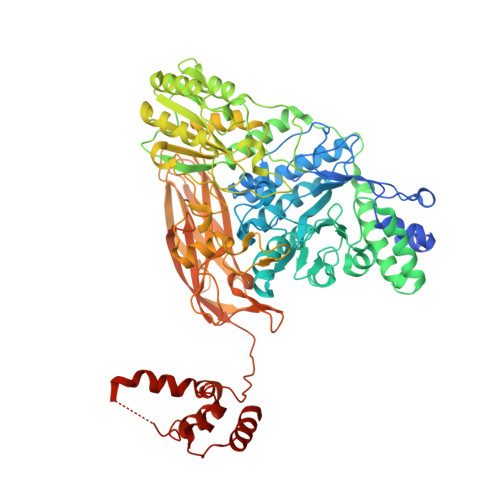
Structural insights into a bacterial beta-glucosidase capable of degrading sesaminol triglucoside to produce sesaminol: toward the understanding of the aglycone recognition mechanism by the C-terminal lid domain.
Yanai, T., Takahashi, Y., Katsumura, E., Sakai, N., Takeshita, K., Imaizumi, R., Matsuura, H., Hongo, S., Waki, T., Takahashi, S., Yamamoto, M., Kataoka, K., Nakayama, T., Yamashita, S.(2023) J Biochem 174: 335-344
- PubMed: 37384427
- DOI: https://doi.org/10.1093/jb/mvad048
- Primary Citation of Related Structures:
8J9F - PubMed Abstract:
The sesaminol triglucoside (STG)-hydrolyzing β-glucosidase from Paenibacillus sp. (PSTG1), which belongs to glycoside hydrolase family 3 (GH3), is a promising catalyst for the industrial production of sesaminol. We determined the X-ray crystal structure of PSTG1 with bound glycerol molecule in the putative active site. PSTG1 monomer contained typical three domains of GH3 with the active site in domain 1 (TIM barrel). In addition, PSTG1 contained an additional domain (domain 4) at the C-terminus that interacts with the active site of the other protomer as a lid in the dimer unit. Interestingly, the interface of domain 4 and the active site forms a hydrophobic cavity probably for recognizing the hydrophobic aglycone moiety of substrate. The short flexible loop region of TIM barrel was found to be approaching the interface of domain 4 and the active site. We found that n-heptyl-β-D-thioglucopyranoside detergent acts as an inhibitor for PSTG1. Thus, we propose that the recognition of hydrophobic aglycone moiety is important for PSTG1-catalyzed reactions. Domain 4 might be a potential target for elucidating the aglycone recognition mechanism of PSTG1 as well as for engineering PSTG1 to create a further excellent enzyme to degrade STG more efficiently to produce sesaminol.
- Graduate School of Natural Science and Technology, Kanazawa University, Kakuma, Kanazawa 920-1192, Japan.
Organizational Affiliation: