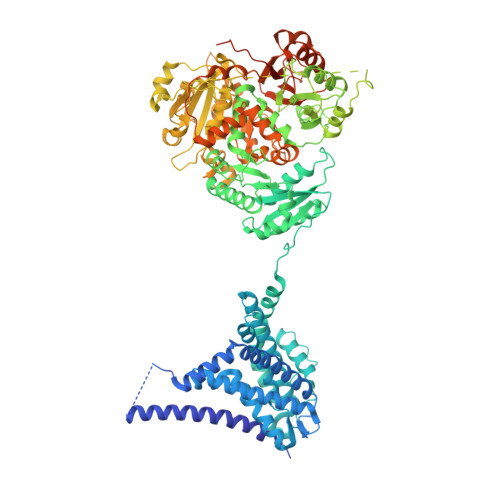
Cryo-EM structure of the Slo1 potassium channel with the auxiliary gamma 1 subunit suggests a mechanism for depolarization-independent activation.
Redhardt, M., Raunser, S., Raisch, T.(2024) FEBS Lett 598: 875-888
- PubMed: 38553946
- DOI: https://doi.org/10.1002/1873-3468.14863
- Primary Citation of Related Structures:
8S3E - PubMed Abstract:
Mammalian Ca 2+ -dependent Slo K + channels can stably associate with auxiliary γ subunits which fundamentally alter their behavior. By a so far unknown mechanism, the four γ subunits reduce the need for voltage-dependent activation and, thereby, allow Slo to open independently of an action potential. Here, using cryo-EM, we reveal how the transmembrane helix of γ1/LRRC26 binds and presumably stabilizes the activated voltage-sensor domain of Slo1. The activation is further enhanced by an intracellular polybasic stretch which locally changes the charge gradient across the membrane. Our data provide a possible explanation for Slo1 regulation by the four γ subunits and also their different activation efficiencies. This suggests a novel activation mechanism of voltage-gated ion channels by auxiliary subunits.
- Department of Structural Biochemistry, Max Planck Institute of Molecular Physiology, Dortmund, Germany.
Organizational Affiliation: