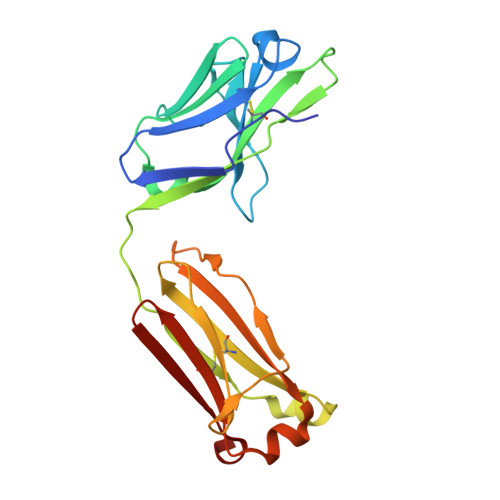
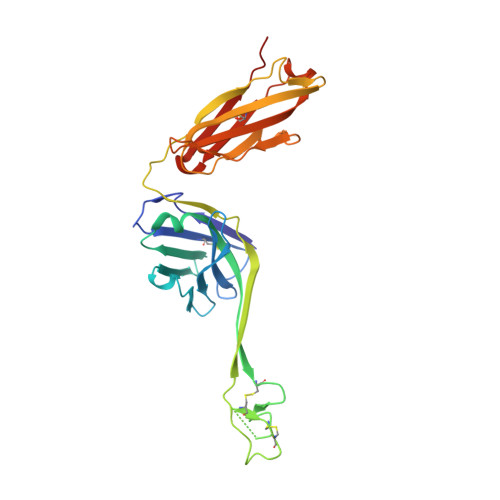
Immunization of cows with HIV envelope trimers generates broadly neutralizing antibodies to the V2-apex from the ultralong CDRH3 repertoire.
Altman, P.X., Ozorowski, G., Stanfield, R.L., Haakenson, J., Appel, M., Parren, M., Lee, W.H., Sang, H., Woehl, J., Saye-Francisco, K., Sewall, L.M., Joyce, C., Song, G., Porter, K., Landais, E., Andrabi, R., Wilson, I.A., Ward, A.B., Mwangi, W., Smider, V.V., Burton, D.R., Sok, D.(2024) PLoS Pathog 20: e1012042-e1012042
- PubMed: 39250525
- DOI: https://doi.org/10.1371/journal.ppat.1012042
- Primary Citation of Related Structures:
8TQ1, 8V4I, 8VBJ, 8VBK, 8VBL, 8VBM, 8VBN, 8VBO, 8VBP, 8VBQ, 8VBR - PubMed Abstract:
The generation of broadly neutralizing antibodies (bnAbs) to conserved epitopes on HIV Envelope (Env) is one of the cornerstones of HIV vaccine research. The animal models commonly used for HIV do not reliably produce a potent broadly neutralizing serum antibody response, with the exception of cows. Cows have previously produced a CD4 binding site response by homologous prime and boosting with a native-like Env trimer. In small animal models, other engineered immunogens were shown to focus antibody responses to the bnAb V2-apex region of Env. Here, we immunized two groups of cows (n = 4) with two regimens of V2-apex focusing Env immunogens to investigate whether antibody responses could be generated to the V2-apex on Env. Group 1 was immunized with chimpanzee simian immunodeficiency virus (SIV)-Env trimer that shares its V2-apex with HIV, followed by immunization with C108, a V2-apex focusing immunogen, and finally boosted with a cross-clade native-like trimer cocktail. Group 2 was immunized with HIV C108 Env trimer followed by the same HIV trimer cocktail as Group 1. Longitudinal serum analysis showed that one cow in each group developed serum neutralizing antibody responses to the V2-apex. Eight and 11 bnAbs were isolated from Group 1 and Group 2 cows, respectively, and showed moderate breadth and potency. Potent and broad responses in this study developed much later than previous cow immunizations that elicited CD4bs bnAbs responses and required several different immunogens. All isolated bnAbs were derived from the ultralong CDRH3 repertoire. The finding that cow antibodies can target more than one broadly neutralizing epitope on the HIV surface reveals the generality of elongated structures for the recognition of highly glycosylated proteins. The exclusive isolation of ultralong CDRH3 bnAbs, despite only comprising a small percent of the cow repertoire, suggests these antibodies outcompete the long and short CDRH3 antibodies during the bnAb response.
- Department of Immunology and Microbiology, The Scripps Research Institute, La Jolla, California, United States of America.
Organizational Affiliation: