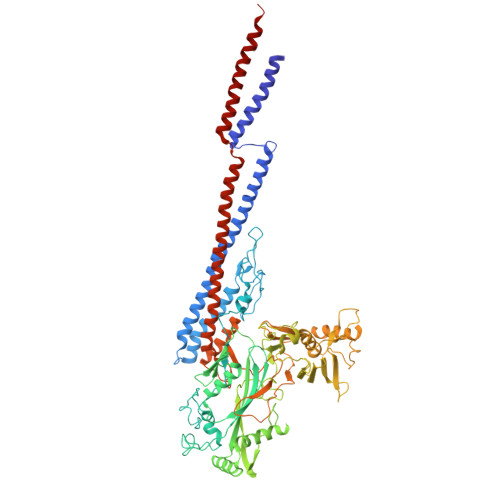
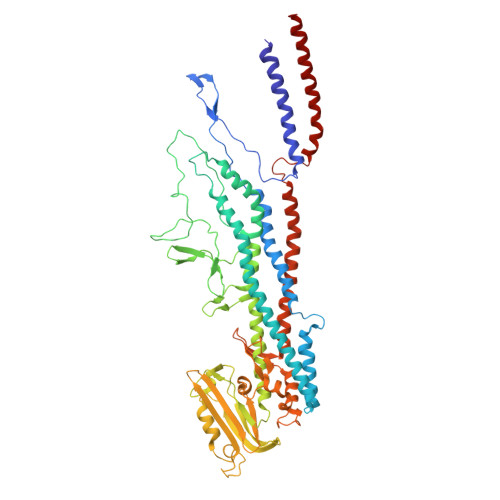
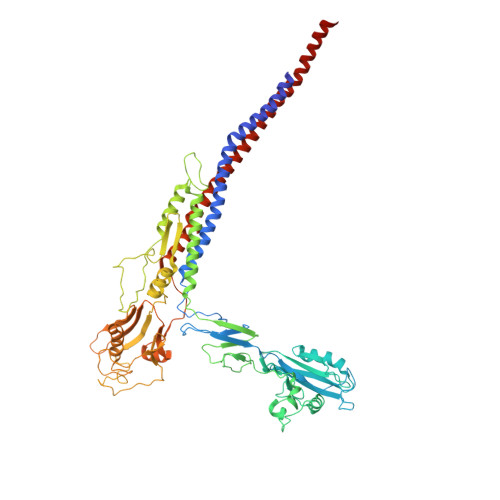
The structure of the complete extracellular bacterial flagellum reveals the mechanism of flagellin incorporation.
Einenkel, R., Qin, K., Schmidt, J., Al-Otaibi, N.S., Mann, D., Drobnic, T., Cohen, E.J., Gonzalez-Rodriguez, N., Harrowell, J., Shmakova, E., Beeby, M., Erhardt, M., Bergeron, J.R.C.(2025) Nat Microbiol 10: 1741-1757
- PubMed: 40595287
- DOI: https://doi.org/10.1038/s41564-025-02037-0
- Primary Citation of Related Structures:
9GNZ, 9GO6, 9GSX - PubMed Abstract:
The bacterial flagellum is essential for motility, adhesion and colonization in pathogens such as Salmonella enterica and Campylobacter jejuni. Its extracellular structure comprises the hook, hook-filament junction, filament and filament cap. Native structures of the hook-filament junction and the cap are lacking, and molecular mechanisms of cap-mediated filament assembly are largely uncharacterized. Here we use cryo-electron microscopy to resolve structures of the complete Salmonella extracellular flagellum including the pentameric FliD cap complex (3.7 Å) and the FlgKL hook-filament junction (2.9 Å), as well as the Campylobacter extracellular flagellum before filament assembly (6.5 Å). This, coupled with structure-guided mutagenesis and functional assays, reveals intermediates of filament assembly, showing that FliD cap protein terminal domain movement and clockwise rotation enable flagellin incorporation and stabilization of the filament. We show that the hook-filament junction acts as a buffer, preventing transfer of mechanical stress to the filament, and reveal the structural basis for the initiation of filament assembly. Collectively, this study provides comprehensive insights into flagellum assembly and how flagellin incorporation is coupled with its secretion.
- Institute of Biology, Humboldt-Universität zu Berlin, Berlin, Germany.
Organizational Affiliation: