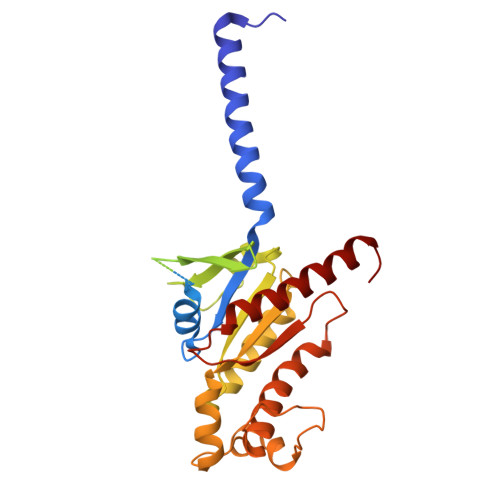
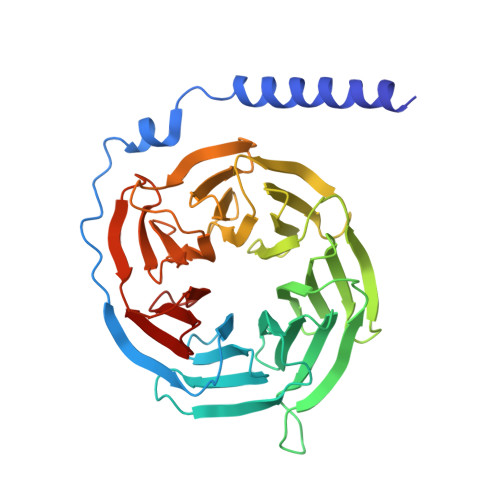
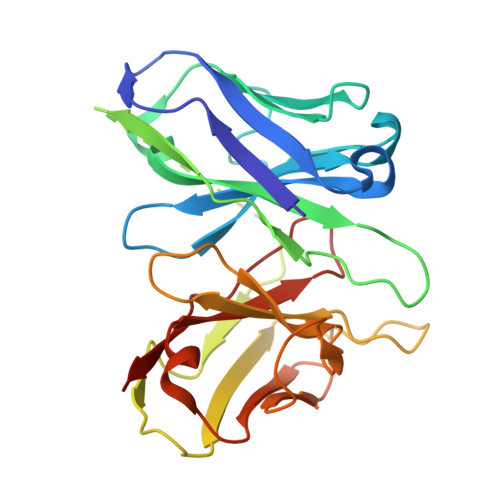
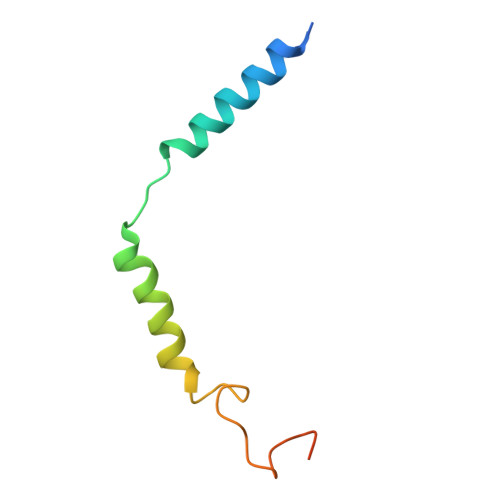
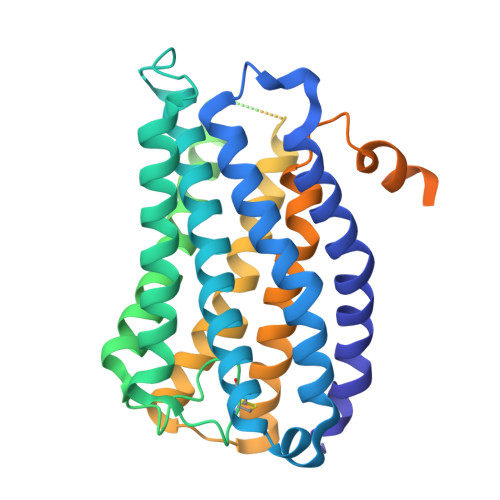
Cryo-EM structures of GPR75 reveal an occluded orthosteric pocket challenging conventional drug discovery paradigms for an anti-obesity target.
Zhu, Z.N., You, C.Z., Yuan, Q.N., Xu, J.Y., Gu, Z.Y., Huang, Z., Liu, M., Shan, B., Wang, J.J., Hu, W., Wang, K., Yin, W.C., Xu, Y.W., Xu, H.E., Wu, C.R.(2026) Acta Pharmacol Sin
- PubMed: 41545757
- DOI: https://doi.org/10.1038/s41401-025-01720-6
- Primary Citation of Related Structures:
9XQC, 9XQN - PubMed Abstract:
The global obesity epidemic, affecting over 650 million adults, demands innovative therapeutics. GPR75 has emerged as a promising anti-obesity target, with genetic evidence linking loss-of-function variants to protection against obesity and type 2 diabetes. However, structural insights have remained elusive due to GPR75's inherent expression and stabilization challenges. Here we present the cryo-EM structures of human GPR75 in apo and Gq-coupled states, achieved through advanced stabilization techniques including NanoBiT and molecular glue approaches. Our structures reveal unique architectural features: a completely collapsed extracellular domain eliminates the traditional orthosteric binding pocket, raising critical questions about previously reported small molecule ligands. GPR75 assumes active-like conformation in both apo and G protein complexed structures through unique molecular switches-the canonical DRY motif is replaced by HRL, abolishing the ionic lock, while a distinctive Lys134-Asp210 salt bridge stabilizes the active conformation without ligand binding. This dramatic structural divergence from conventional GPCRs necessitates alternative therapeutic strategies targeting allosteric sites or protein-protein interactions rather than orthosteric pockets. Our findings establish a crucial structural framework for developing next-generation anti-obesity therapeutics.
- State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203, China.
Organizational Affiliation: