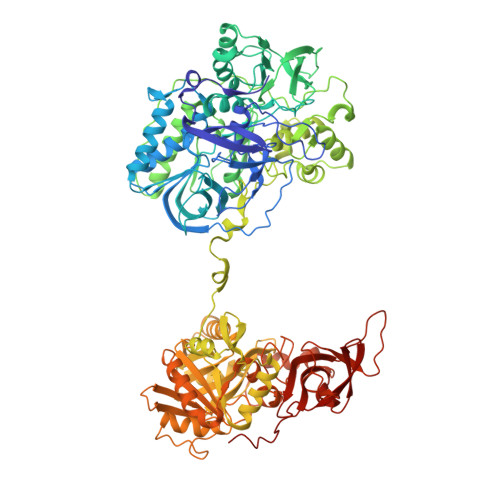
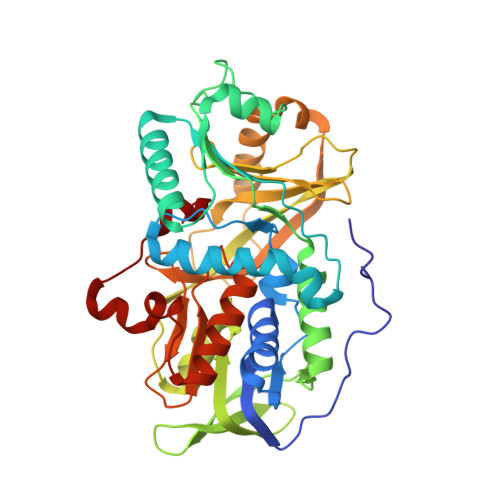
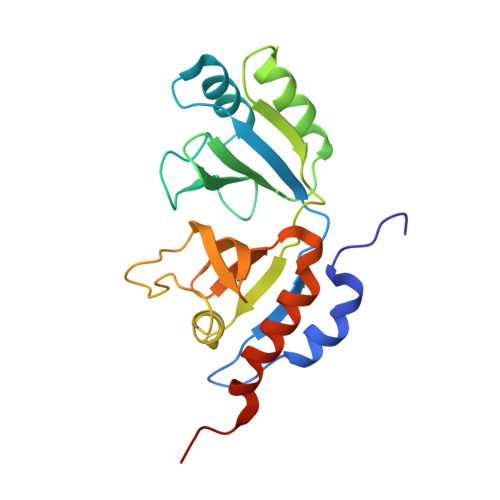
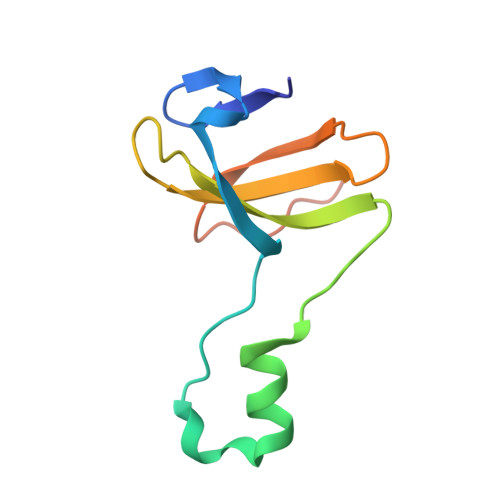
Channeling and conformational changes in the heterotetrameric sarcosine oxidase from Corynebacterium sp. U-96.
Moriguchi, T., Ida, K., Hikima, T., Ueno, G., Yamamoto, M., Suzuki, H.(2010) J Biochem 148: 491-505
- PubMed: 20675294
- DOI: https://doi.org/10.1093/jb/mvq083
- Primary Citation of Related Structures:
3AD7, 3AD8, 3AD9, 3ADA - PubMed Abstract:
We characterized the crystal structures of heterotetrameric sarcosine oxidase (SO) from Corynebacterium sp. U-96 complexed with methylthioacetate (MTA), pyrrole 2-carboxylate (PCA) and sulphite, and of sarcosine-reduced SO. SO comprises α-, β-, γ- and δ-subunits; FAD and FMN cofactors; and a large internal cavity. MTA and PCA are sandwiched between the re-face of the FAD isoalloxazine ring and the β-subunit C-terminal residues. Reduction of flavin cofactors shifts the β-subunit Ala1 towards the α-subunit Met55, forming a surface cavity at the oxygen-channel vestibule and rendering the β-subunit C-terminal residues mobile. We identified three channels connecting the cavity and the enzyme surface. Two of them exist in the inter-subunit space between α and β-subunits, and the substrate sarcosine seems to enter the active site through either of these channels and reaches the re-side of the FAD isoalloxazine ring by traversing the mobile β-subunit C-terminal residues. The third channel goes through the α-subunit and has a folinic acid-binding site, where the iminium intermediate is converted to Gly and either formaldehyde or, 5,10-methylenetetrahydrofolate. Oxygen molecules are probably located on the surface cavity and diffuse to the FMN isoalloxazine ring; the H(2)O(2) formed exits via the oxygen channel.
Organizational Affiliation:
Division of Bioscience, Graduate School of Fundamental Life Science, Kitasato University, Kitasato 1-15-1, Sagamihara, Kanagawa 252-0329, Japan.