Modular evolution and design of the protein binding interface
Lee, J., Kim, H.J., Yang, C., Choi, J., Kyeong, H., Yuk, J., Park, K., Kim, Y.J., Heu, W., Lee, S., Kim, D., Jo, E.K., Cheong, H.K., Kim, H.S.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Internalin B,REPEAT MODULES,Variable lymphocyte receptor B | 267 | Listeria monocytogenes, synthetic construct, Eptatretus burgeri This entity is chimeric | Mutation(s): 0 | 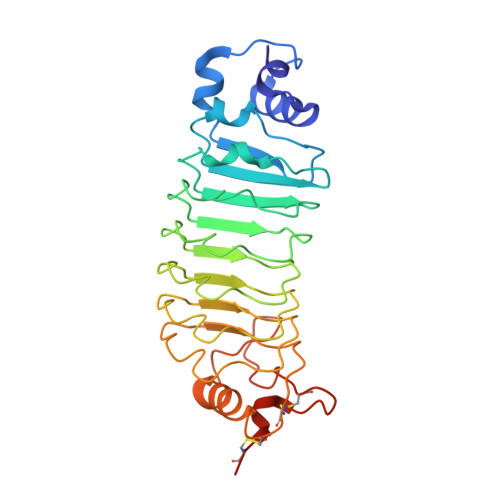 | |
UniProt | |||||
Find proteins for A4L9V2 (Listeria monocytogenes) Explore A4L9V2 Go to UniProtKB: A4L9V2 | |||||
Find proteins for Q4G1L3 (Eptatretus burgeri) Explore Q4G1L3 Go to UniProtKB: Q4G1L3 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Groups | Q4G1L3A4L9V2 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Interleukin-6 | 168 | Homo sapiens | Mutation(s): 0 Gene Names: IL6, IFNB2 | 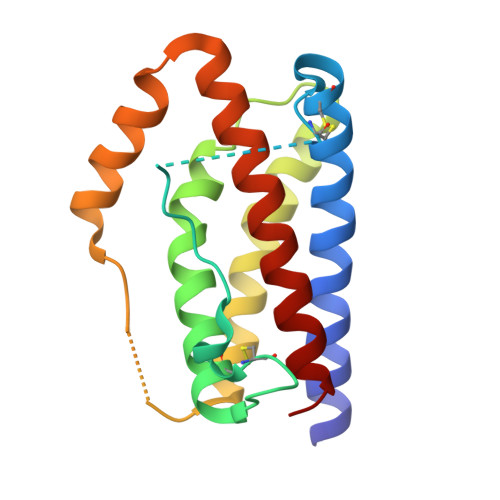 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P05231 (Homo sapiens) Explore P05231 Go to UniProtKB: P05231 | |||||
PHAROS: P05231 GTEx: ENSG00000136244 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P05231 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 46.319 | α = 90 |
| b = 134.001 | β = 90 |
| c = 148.722 | γ = 90 |
| Software Name | Purpose |
|---|---|
| ADSC | data collection |
| CNS | refinement |
| REFMAC | refinement |
| MOSFLM | data reduction |
| SCALA | data scaling |
| CNS | phasing |