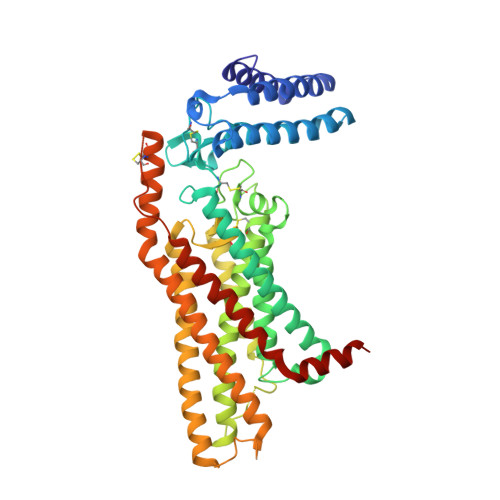
Structure of the human smoothened receptor bound to an antitumour agent.
Wang, C., Wu, H., Katritch, V., Han, G.W., Huang, X.P., Liu, W., Siu, F.Y., Roth, B.L., Cherezov, V., Stevens, R.C.(2013) Nature 497: 338-343
- PubMed: 23636324
- DOI: https://doi.org/10.1038/nature12167
- Primary Citation of Related Structures:
4JKV - PubMed Abstract:
The smoothened (SMO) receptor, a key signal transducer in the hedgehog signalling pathway, is responsible for the maintenance of normal embryonic development and is implicated in carcinogenesis. It is classified as a class frizzled (class F) G-protein-coupled receptor (GPCR), although the canonical hedgehog signalling pathway involves the GLI transcription factors and the sequence similarity with class A GPCRs is less than 10%. Here we report the crystal structure of the transmembrane domain of the human SMO receptor bound to the small-molecule antagonist LY2940680 at 2.5 Å resolution. Although the SMO receptor shares the seven-transmembrane helical fold, most of the conserved motifs for class A GPCRs are absent, and the structure reveals an unusually complex arrangement of long extracellular loops stabilized by four disulphide bonds. The ligand binds at the extracellular end of the seven-transmembrane-helix bundle and forms extensive contacts with the loops.
- Department of Integrative Structural and Computational Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, USA.
Organizational Affiliation: