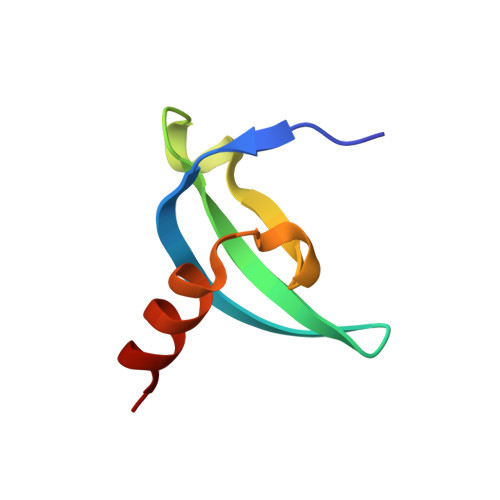
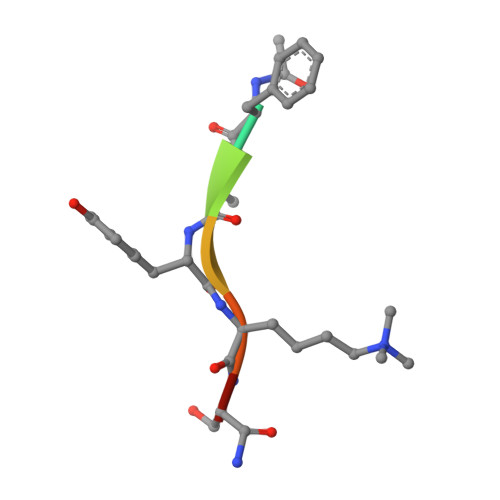
Chromodomain Antagonists That Target the Polycomb-Group Methyllysine Reader Protein Chromobox Homolog 7 (CBX7).
Simhadri, C., Daze, K.D., Douglas, S.F., Quon, T.T., Dev, A., Gignac, M.C., Peng, F., Heller, M., Boulanger, M.J., Wulff, J.E., Hof, F.(2014) J Med Chem 57: 2874-2883
- PubMed: 24625057
- DOI: https://doi.org/10.1021/jm401487x
- Primary Citation of Related Structures:
4MN3 - PubMed Abstract:
We report here a peptide-driven approach to create first inhibitors of the chromobox homolog 7 (CBX7), a methyllysine reader protein. CBX7 uses its chromodomain to bind histone 3, lysine 27 trimethylated (H3K27me3), and this recognition event is implicated in silencing multiple tumor suppressors. Small trimethyllysine containing peptides were used as the basic scaffold from which potent ligands for disruption of CBX7-H3K27me3 complex were developed. Potency of ligands was determined by fluorescence polarization and/or isothermal titration calorimetry. Binding of one ligand was characterized in detail using 2D NMR and X-ray crystallography, revealing a structural motif unique among human CBX proteins. Inhibitors with a ∼200 nM potency for CBX7 binding and 10-fold/400-fold selectivity over related CBX8/CBX1 proteins were identified. These are the first reported inhibitors of any chromodomain.
- Department of Chemistry, University of Victoria , P.O. Box 3065, Victoria, British Columbia, V8W 3V6, Canada.
Organizational Affiliation: