Structural and Biochemical Features of Eimeria tenella Dihydroorotate Dehydrogenase, a Potential Drug Target.
Sato, D., Hartuti, E.D., Inaoka, D.K., Sakura, T., Amalia, E., Nagahama, M., Yoshioka, Y., Tsuji, N., Nozaki, T., Kita, K., Harada, S., Matsubayashi, M., Shiba, T.(2020) Genes (Basel) 11
- PubMed: 33297567
- DOI: https://doi.org/10.3390/genes11121468
- Primary Citation of Related Structures:
6AJ5, 6AJE, 6IDJ - PubMed Abstract:
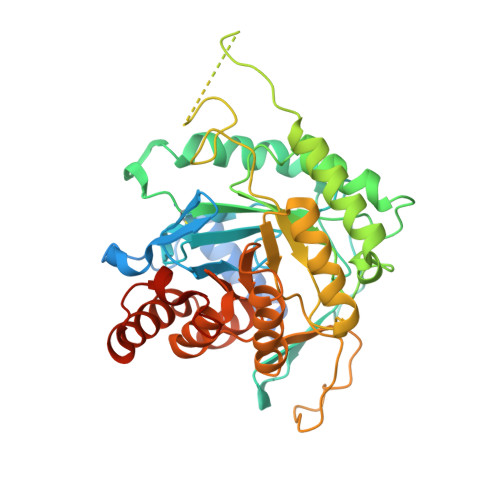
Dihydroorotate dehydrogenase (DHODH) is a mitochondrial monotopic membrane protein that plays an essential role in the pyrimidine de novo biosynthesis and electron transport chain pathways. In Eimeria tenella , an intracellular apicomplexan parasite that causes the most severe form of chicken coccidiosis, the activity of pyrimidine salvage pathway at the intracellular stage is negligible and it relies on the pyrimidine de novo biosynthesis pathway. Therefore, the enzymes of the de novo pathway are considered potential drug target candidates for the design of compounds with activity against this parasite. Although, DHODHs from E. tenella (EtDHODH), Plasmodium falciparum (PfDHODH), and human (HsDHODH) show distinct sensitivities to classical DHODH inhibitors, in this paper, we identify ferulenol as a potent inhibitor of both EtDHODH and HsDHODH. Additionally, we report the crystal structures of EtDHODH and HsDHODH in the absence and presence of ferulenol. Comparison of these enzymes showed that despite similar overall structures, the EtDHODH has a long insertion in the N-terminal helix region that assumes a disordered configuration. In addition, the crystal structures revealed that the ferulenol binding pocket of EtDHODH is larger than that of HsDHODH. These differences can be explored to accelerate structure-based design of inhibitors specifically targeting EtDHODH.
- Department of Applied Biology, Graduate School of Science Technology, Kyoto Institute of Technology, Matsugasaki, Sakyo-ku, Kyoto 606-8585, Japan.
Organizational Affiliation: