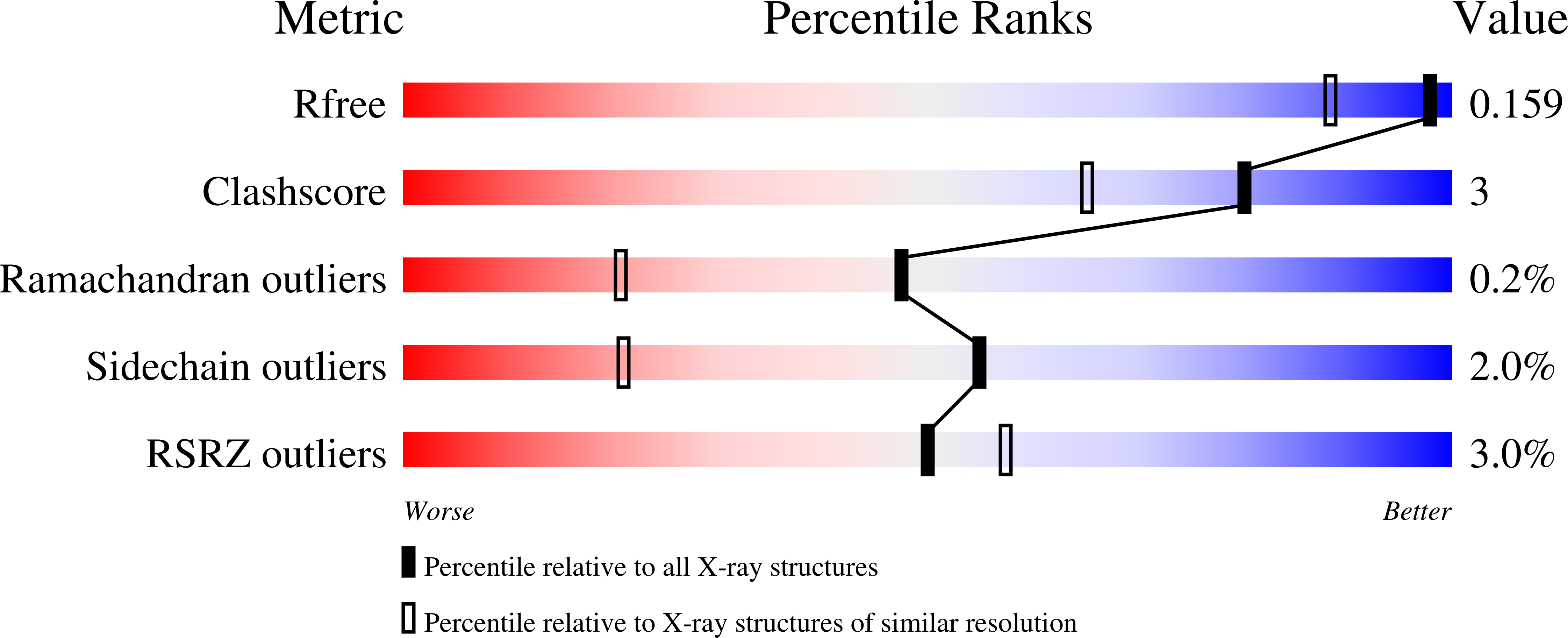
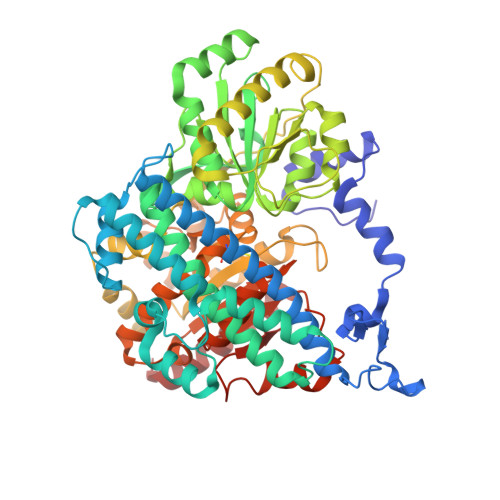
A Morphing [4Fe-3S-nO]-Cluster within a Carbon Monoxide Dehydrogenase Scaffold.
Jeoung, J.H., Fesseler, J., Domnik, L., Klemke, F., Sinnreich, M., Teutloff, C., Dobbek, H.(2022) Angew Chem Int Ed Engl 61: e202117000-e202117000
- PubMed: 35133707
- DOI: https://doi.org/10.1002/anie.202117000
- Primary Citation of Related Structures:
7B7Q, 7B7T, 7B95, 7B97, 7B9A - PubMed Abstract:
Ni,Fe-containing carbon monoxide dehydrogenases (CODHs) catalyze the reversible reduction of CO 2 to CO. Several anaerobic microorganisms encode multiple CODHs in their genome, of which some, despite being annotated as CODHs, lack a cysteine of the canonical binding motif for the active site Ni,Fe-cluster. Here, we report on the structure and reactivity of such a deviant enzyme, termed CooS-V Ch . Its structure reveals the typical CODH scaffold, but contains an iron-sulfur-oxo hybrid-cluster. Although closely related to true CODHs, CooS-V Ch catalyzes neither CO oxidation, nor CO 2 reduction. The active site of CooS-V Ch undergoes a redox-dependent restructuring between a reduced [4Fe-3S]-cluster and an oxidized [4Fe-2S-S*-2O-2(H 2 O)]-cluster. Hydroxylamine, a slow-turnover substrate of CooS-V Ch , oxidizes the hybrid-cluster in two structurally distinct steps. Overall, minor changes in CODHs are sufficient to accommodate a Fe/S/O-cluster in place of the Ni,Fe-heterocubane-cluster of CODHs.
Organizational Affiliation:
Humboldt-Universität zu Berlin, Institut für Biologie, Unter den Linden 6, 10099, Berlin, Germany.