Comprehensive Assessment of the Relationship Between Site -2 Specificity and Helix alpha 2 in the Erbin PDZ Domain.
Teyra, J., McLaughlin, M., Singer, A., Kelil, A., Ernst, A., Sicheri, F., Sidhu, S.S.(2021) J Mol Biol 433: 167115-167115
- PubMed: 34171344
- DOI: https://doi.org/10.1016/j.jmb.2021.167115
- Primary Citation of Related Structures:
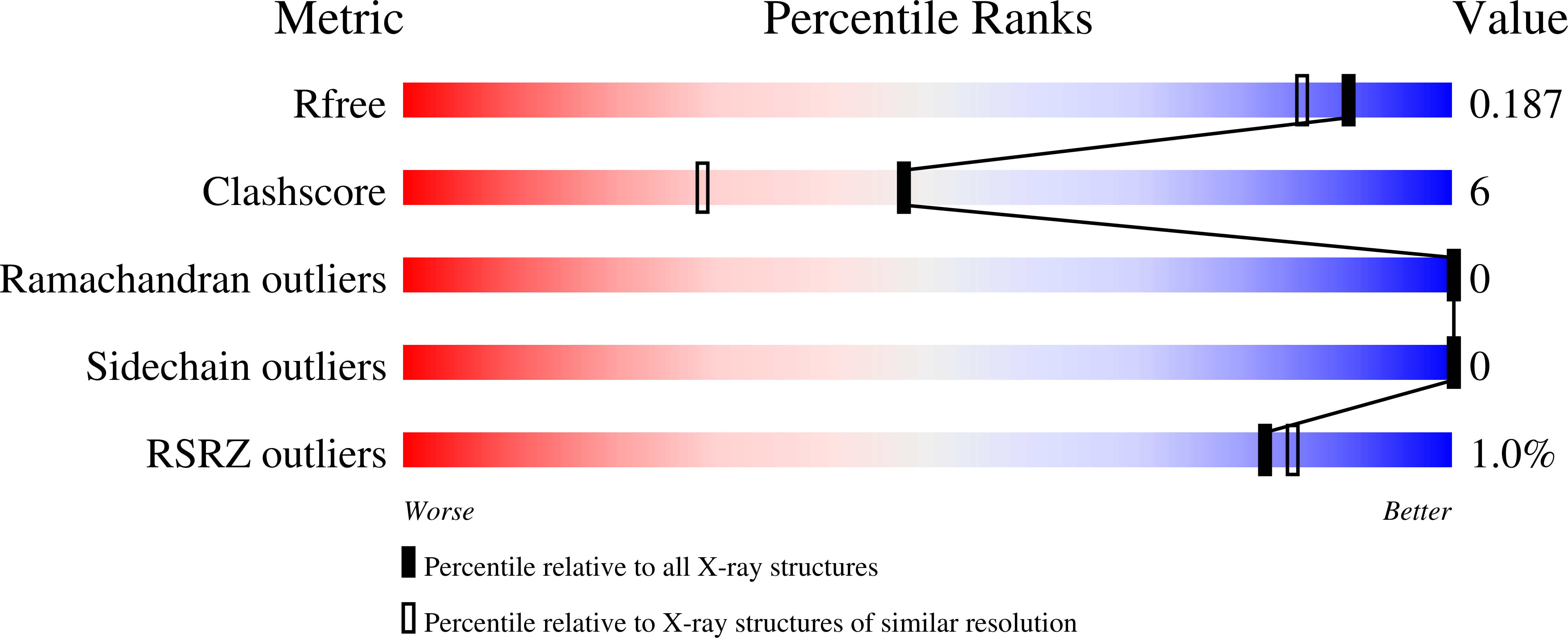
6UBH, 7LUL - PubMed Abstract:
PDZ domains are key players in signalling pathways. These modular domains generally recognize short linear C-terminal stretches of sequences in proteins that organize the formation of complex multi-component assemblies. The development of new methodologies for the characterization of the molecular principles governing these interactions is critical to fully understand the functional diversity of the family and to elucidate biological functions for family members. Here, we applied an in vitro evolution strategy to explore comprehensively the capacity of PDZ domains for specific recognition of different amino acids at a key position in C-terminal peptide ligands. We constructed a phage-displayed library of the Erbin PDZ domain by randomizing the binding site -2 and adjacent residues, which are all contained in helix α2, and we selected for variants binding to a panel of peptides representing all possible position -2 residues. This approach generated insights into the basis for the common natural class I and II specificities, demonstrated an alternative basis for a rare natural class III specificity for Asp -2 , and revealed a novel specificity for Arg -2 that has not been reported in natural PDZ domains. A structure of a PDZ-peptide complex explained the minimum requirement for switching specificity from class I ligands containing Thr/Ser -2 to class II ligands containing hydrophobic residues at position -2 . A second structure explained the molecular basis for the specificity for ligands containing Arg -2 . Overall, the evolved PDZ variants greatly expand our understanding of site -2 specificities and the variants themselves may prove useful as building blocks for synthetic biology.
Organizational Affiliation:
The Donnelly Centre, University of Toronto, Toronto, Ontario, Canada.