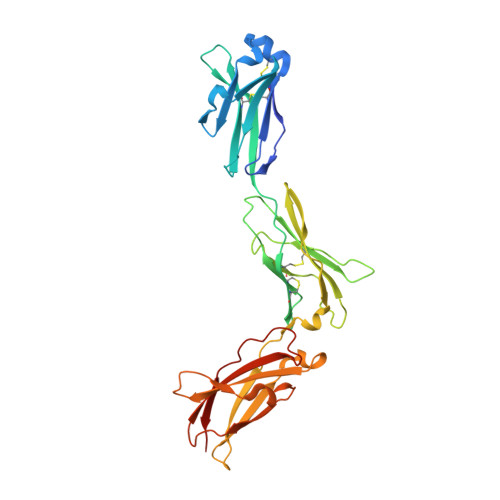
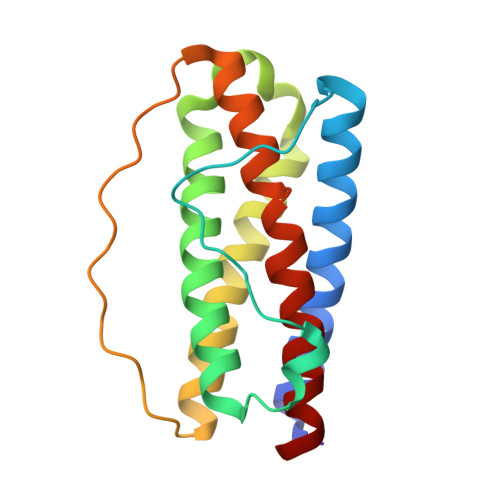
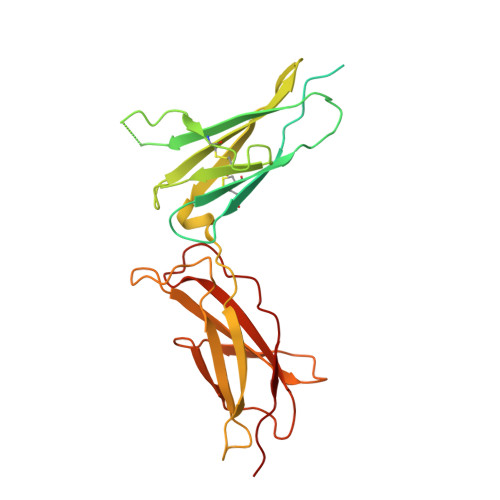
Structures of the interleukin 11 signalling complex reveal gp130 dynamics and the inhibitory mechanism of a cytokine variant.
Metcalfe, R.D., Hanssen, E., Fung, K.Y., Aizel, K., Kosasih, C.C., Zlatic, C.O., Doughty, L., Morton, C.J., Leis, A.P., Parker, M.W., Gooley, P.R., Putoczki, T.L., Griffin, M.D.W.(2023) Nat Commun 14: 7543-7543
- PubMed: 37985757
- DOI: https://doi.org/10.1038/s41467-023-42754-w
- Primary Citation of Related Structures:
8DPS, 8DPT, 8DPU, 8DPV, 8DPW - PubMed Abstract:
Interleukin (IL-)11, an IL-6 family cytokine, has pivotal roles in autoimmune diseases, fibrotic complications, and solid cancers. Despite intense therapeutic targeting efforts, structural understanding of IL-11 signalling and mechanistic insights into current inhibitors are lacking. Here we present cryo-EM and crystal structures of the human IL-11 signalling complex, including the complex containing the complete extracellular domains of the shared IL-6 family β-receptor, gp130. We show that complex formation requires conformational reorganisation of IL-11 and that the membrane-proximal domains of gp130 are dynamic. We demonstrate that the cytokine mutant, IL-11 Mutein, competitively inhibits signalling in human cell lines. Structural shifts in IL-11 Mutein underlie inhibition by altering cytokine binding interactions at all three receptor-engaging sites and abrogating the final gp130 binding step. Our results reveal the structural basis of IL-11 signalling, define the molecular mechanisms of an inhibitor, and advance understanding of gp130-containing receptor complexes, with potential applications in therapeutic development.
- Department of Biochemistry and Pharmacology, Bio21 Molecular Science and Biotechnology Institute, University of Melbourne, Parkville, Victoria, 3010, Australia.
Organizational Affiliation: