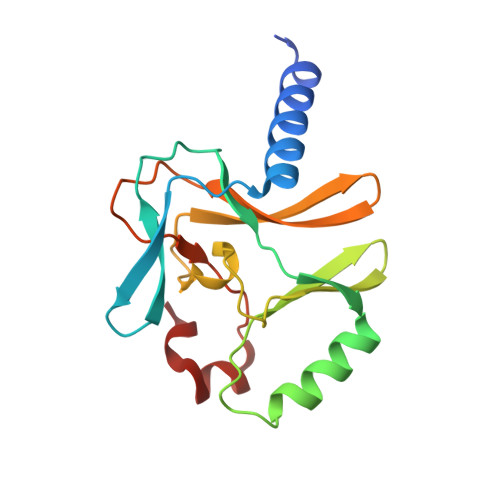
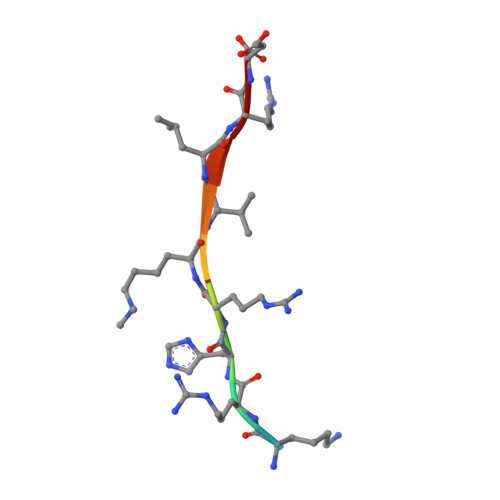
Structural origins for the product specificity of SET domain protein methyltransferases.
Couture, J.F., Dirk, L.M., Brunzelle, J.S., Houtz, R.L., Trievel, R.C.(2008) Proc Natl Acad Sci U S A 105: 20659-20664
- PubMed: 19088188
- DOI: https://doi.org/10.1073/pnas.0806712105
- Primary Citation of Related Structures:
3F9W, 3F9X, 3F9Y, 3F9Z - PubMed Abstract:
SET domain protein lysine methyltransferases (PKMTs) regulate transcription and other cellular functions through site-specific methylation of histones and other substrates. PKMTs catalyze the formation of monomethylated, dimethylated, or trimethylated products, establishing an additional hierarchy with respect to methyllysine recognition in signaling. Biochemical studies of PKMTs have identified a conserved position within their active sites, the Phe/Tyr switch, that governs their respective product specificities. To elucidate the mechanism underlying this switch, we have characterized a Phe/Tyr switch mutant of the histone H4 Lys-20 (H4K20) methyltransferase SET8, which alters its specificity from a monomethyltransferase to a dimethyltransferase. The crystal structures of the SET8 Y334F mutant bound to histone H4 peptides bearing unmodified, monomethyl, and dimethyl Lys-20 reveal that the phenylalanine substitution attenuates hydrogen bonding to a structurally conserved water molecule adjacent to the Phe/Tyr switch, facilitating its dissociation. The additional space generated by the solvent's dissociation enables the monomethyllysyl side chain to adopt a conformation that is catalytically competent for dimethylation and furnishes sufficient volume to accommodate the dimethyl epsilon-ammonium product. Collectively, these results indicate that the Phe/Tyr switch regulates product specificity through altering the affinity of an active-site water molecule whose dissociation is required for lysine multiple methylation.
- Department of Biological Chemistry, University of Michigan, Ann Arbor, MI 48109, USA.
Organizational Affiliation: